A spontaneous multifunctional hydrogel vaccine amplifies the innate immune response to launch a powerful antitumor adaptive immune response
- PMID: 34093863
- PMCID: PMC8171104
- DOI: 10.7150/thno.58173
A spontaneous multifunctional hydrogel vaccine amplifies the innate immune response to launch a powerful antitumor adaptive immune response
Abstract
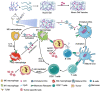
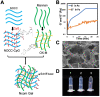
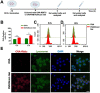
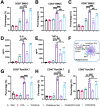
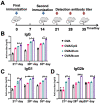
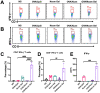
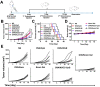
Substantial progress has been made with cancer immunotherapeutic strategies in recent years, most of which mainly rely on enhancing the T cell response. However, sufficient tumor antigen information often cannot be presented to T cells, resulting in a failed effector T cell response. The innate immune system can effectively recognize tumor antigens and then initiate an adaptive immune response. Here, we developed a spontaneous multifunctional hydrogel (NOCC-CpG/OX-M, Ncom Gel) vaccine to amplify the innate immune response and harness innate immunity to launch and maintain a powerful adaptive immune response. Methods: Ncom Gel was formed by a Schiff base reaction between CpG-modified carboxymethyl chitosan (NOCC-CpG) and partially oxidized mannan (OX-M). The effects of the Ncom Gel vaccine on DCs and macrophages in vitro and antigen-specific humoral immunity and cellular immunity in vivo were studied. Furthermore, the antitumor immune response of the Ncom Gel vaccine and its effect on the tumor microenvironment were evaluated. Results: The Ncom Gel vaccine enhanced antigen presentation to T cells by facilitating DC uptake and maturation and inducing macrophages to a proinflammatory subtype, further leading to a T cell-mediated adaptive immune response. Moreover, the innate immune response could be amplified via the promotion of antigen-specific antibody production. The Ncom Gel vaccine reversed the tumor immune microenvironment to an inflamed phenotype and showed a significant antitumor response in a melanoma model. Conclusions: Our research implies the potential application of injectable hydrogels as a platform for tumor immunotherapy. The strategy also opens up a new avenue for multilayered cancer immunotherapy.
Keywords: Ncom Gel vaccine; adaptive immune response; cancer immunotherapy; innate immunity; spontaneous multifunctional hydrogel.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. - PubMed
-
- Phuengkham H, Ren L, Shin IW, Lim YT. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv Mater. 2019;31:1803322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

