The metabesity factor HMG20A potentiates astrocyte survival and reactive astrogliosis preserving neuronal integrity
- PMID: 34093866
- PMCID: PMC8171100
- DOI: 10.7150/thno.57237
The metabesity factor HMG20A potentiates astrocyte survival and reactive astrogliosis preserving neuronal integrity
Abstract
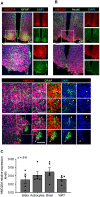
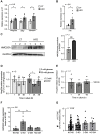
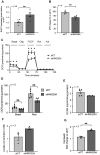
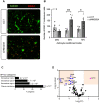
Rationale: We recently demonstrated that the 'Metabesity' factor HMG20A regulates islet beta-cell functional maturity and adaptation to physiological stress such as pregnancy and pre-diabetes. HMG20A also dictates central nervous system (CNS) development via inhibition of the LSD1-CoREST complex but its expression pattern and function in adult brain remains unknown. Herein we sought to determine whether HMG20A is expressed in the adult CNS, specifically in hypothalamic astrocytes that are key in glucose homeostasis and whether similar to islets, HMG20A potentiates astrocyte function in response to environmental cues. Methods: HMG20A expression profile was assessed by quantitative PCR (QT-PCR), Western blotting and/or immunofluorescence in: 1) the hypothalamus of mice exposed or not to either a high-fat diet or a high-fat high-sucrose regimen, 2) human blood leukocytes and adipose tissue obtained from healthy or diabetic individuals and 3) primary mouse hypothalamic astrocytes exposed to either high glucose or palmitate. RNA-seq and cell metabolic parameters were performed on astrocytes treated or not with a siHMG20A. Astrocyte-mediated neuronal survival was evaluated using conditioned media from siHMG20A-treated astrocytes. The impact of ORY1001, an inhibitor of the LSD1-CoREST complex, on HMG20A expression, reactive astrogliosis and glucose metabolism was evaluated in vitro and in vivo in high-fat high-sucrose fed mice. Results: We show that Hmg20a is predominantly expressed in hypothalamic astrocytes, the main nutrient-sensing cell type of the brain. HMG20A expression was upregulated in diet-induced obesity and glucose intolerant mice, correlating with increased transcript levels of Gfap and Il1b indicative of inflammation and reactive astrogliosis. Hmg20a transcript levels were also increased in adipose tissue of obese non-diabetic individuals as compared to obese diabetic patients. HMG20A silencing in astrocytes resulted in repression of inflammatory, cholesterol biogenesis and epithelial-to-mesenchymal transition pathways which are hallmarks of reactive astrogliosis. Accordingly, HMG20A depleted astrocytes exhibited reduced mitochondrial bioenergetics and increased susceptibility to apoptosis. Neuron viability was also hindered in HMG20A-depleted astrocyte-derived conditioned media. ORY1001 treatment rescued expression of reactive astrogliosis-linked genes in HMG20A ablated astrocytes while enhancing cell surface area, GFAP intensity and STAT3 expression in healthy astrocytes, mimicking the effect of HMG20A. Furthermore, ORY1001 treatment protected against obesity-associated glucose intolerance in mice correlating with a regression of hypothalamic HMG20A expression, indicative of reactive astrogliosis attenuation with improved health status. Conclusion: HMG20A coordinates the astrocyte polarization state. Under physiological pressure such as obesity and insulin resistance that induces low grade inflammation, HMG20A expression is increased to induce reactive astrogliosis in an attempt to preserve the neuronal network and re-establish glucose homeostasis. Nonetheless, a chronic metabesity state or functional mutations will result in lower levels of HMG20A, failure to promote reactive astrogliosis and increase susceptibility of neurons to stress-induced apoptosis. Such effects could be reversed by ORY1001 treatment both in vitro and in vivo, paving the way for a new therapeutic approach for Type 2 Diabetes Mellitus.
Keywords: HMG20A; ORY1001.; astrocytes; inflammation; metabesity; metabolism.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13:435–44. - PubMed
-
- Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13:465–476. - PubMed
-
- Donath MY. Inflammation and type 2 diabetes: from basic science to treatment. Semin Immunopathol. 2019;41:411–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

