An updated systematic review and meta-analysis on Mycobacterium tuberculosis antibiotic resistance in Iran (2013-2020)
- PMID: 34094023
- PMCID: PMC8143714
- DOI: 10.22038/IJBMS.2021.48628.11161
An updated systematic review and meta-analysis on Mycobacterium tuberculosis antibiotic resistance in Iran (2013-2020)
Abstract
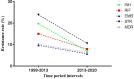
This updated systematic review and meta-analysis follows two aims: 1) to assess Mycobacterium tuberculosis (M. tuberculosis) antibiotic resistance in Iran from 2013 to 2020 and, 2) to assess the trend of resistance from 1999 to 2020. Several national and international databases were systematically searched through MeSH extracted keywords to identify 41 published studies addressing drug-resistant M. tuberculosis in Iran. Meta-analysis was done based on the PRISMA protocols using Comprehensive Meta-Analysis software. The average prevalence of resistance to first- and second-line anti-TB drugs, multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) in new and previously treated tuberculosis (TB) cases in Iran during 2013-2020 were as follows: isoniazid 6.9%, rifampin 7.9%, ethambutol 5.7%, pyrazinamide 20.4%, para-aminosalicylic acid 4.6%, capreomycin 1.7%, cycloserine 1.8%, ethionamide 11.3%, ofloxacin 1.5%, kanamycin 3.8%, amikacin 2.2%, MDR-TB 6.3% and XDR-TB 0.9%. Based on the presented data, M. tuberculosis resistance to first- and second-line anti-TB drugs, as well as MDR-TB, was low during 2013-2020 in Iran. Furthermore, there was a declining trend in TB drug resistance from 1999 to 2020. Hence, to maintain the current decreasing trend and to control and eliminate TB infection in Iran, continuous monitoring of resistance patterns is recommended.
Keywords: Antibiotic; Iran; Meta-analysis; Mycobacterium tuberculosis; Resistance.
Figures

Similar articles
-
Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran.Chest. 2009 Aug;136(2):420-425. doi: 10.1378/chest.08-2427. Epub 2009 Apr 6. Chest. 2009. PMID: 19349380
-
Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a Medical Center in Taiwan.Clin Infect Dis. 2008 Oct 1;47(7):e57-63. doi: 10.1086/591702. Clin Infect Dis. 2008. PMID: 18715157
-
Impact of extensively drug-resistant tuberculosis on treatment outcome of multidrug-resistant tuberculosis patients with standardized regimen: report from Iran.Microb Drug Resist. 2010 Mar;16(1):81-6. doi: 10.1089/mdr.2009.0073. Microb Drug Resist. 2010. PMID: 20192820
-
Overview of anti-tuberculosis (TB) drugs and their resistance mechanisms.Mini Rev Med Chem. 2007 Nov;7(11):1177-85. doi: 10.2174/138955707782331740. Mini Rev Med Chem. 2007. PMID: 18045221 Review.
-
Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis.Lancet Infect Dis. 2010 Sep;10(9):621-9. doi: 10.1016/S1473-3099(10)70139-0. Lancet Infect Dis. 2010. PMID: 20797644 Review.
Cited by
-
Genetic diversity of drug-resistant Mycobacterium tuberculosis clinical isolates from Khuzestan province, Iran.AMB Express. 2022 Jul 4;12(1):85. doi: 10.1186/s13568-022-01425-7. AMB Express. 2022. PMID: 35789443 Free PMC article.
-
Factors Associated with Non-Adherence to Treatment Among Migrants with MDR-TB in Wuhan, China: A Cross-Sectional Study.Risk Manag Healthc Policy. 2024 Mar 26;17:727-737. doi: 10.2147/RMHP.S448706. eCollection 2024. Risk Manag Healthc Policy. 2024. PMID: 38559871 Free PMC article.
-
Molecular identification of multiple drug resistance (MDR) strain of Mycobacterium tuberculosis.Mol Biol Rep. 2023 Dec;50(12):10271-10275. doi: 10.1007/s11033-023-08867-7. Epub 2023 Nov 16. Mol Biol Rep. 2023. PMID: 37971566
-
Drug resistance and genomic variations among Mycobacterium tuberculosis isolates from The Nile Delta, Egypt.Sci Rep. 2024 Sep 2;14(1):20401. doi: 10.1038/s41598-024-70199-8. Sci Rep. 2024. PMID: 39223176 Free PMC article.
-
Epidemiology of first- and second-line drugs-resistant pulmonary tuberculosis in Iran: Systematic review and meta-analysis.J Clin Tuberc Other Mycobact Dis. 2024 Mar 16;35:100430. doi: 10.1016/j.jctube.2024.100430. eCollection 2024 May. J Clin Tuberc Other Mycobact Dis. 2024. PMID: 38560029 Free PMC article. Review.
References
-
- World Health Organization. Global tuberculosis report 2019. Gevena [Switzerland]: World Health Organization; 2019.
-
- Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology. 8th ed. UK: Elsevier Health Sciences: 2015. pp. 221–225.
-
- Carroll KC, Butel JS, Morse SA. Jawetz Melnick & Adelbergs medical microbiology. 27th ed. Pennsylvania: McGraw Hill Professional; 2016. pp. 309–317.
-
- Khademi F, Taheri RA, Avarvand AY, Vaez H, Momtazi-Borojeni AA, Soleimanpour S. Are chitosan natural polymers suitable as adjuvant/delivery system for anti-tuberculosis vaccines? Microb Pathog. 2018;121:218–223. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
