Enhancing the selectivity of prolinamide organocatalysts using the mechanical bond in [2]rotaxanes
- PMID: 34094051
- PMCID: PMC8152698
- DOI: 10.1039/d0sc00444h
Enhancing the selectivity of prolinamide organocatalysts using the mechanical bond in [2]rotaxanes
Abstract
The synthesis of a pair of switchable interlocked prolinamides and their use as organocatalysts in three different enamine-activated processes are reported. A diacylaminopyridine moiety was incorporated into the thread for directing [2]rotaxane formation further allowing the association of complementary small molecules. The rotaxane-based systems were tested as organocatalysts in asymmetric enamine-mediated processes, revealing a significantly improved catalytic ability if compared with the non-interlocked thread. The presence of an electron-withdrawing nitro group at the macrocycle helps to achieve high conversions and enantioselectivities. These systems are able to interact with N-hexylthymine as a cofactor to form supramolecular catalysts displaying a divergent catalytic behaviour. The presence or absence of the cofactor controls the chemoselectivity in competitive reactions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
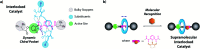





References
-
- Dalko P. I., Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, Wiley, Weinheim, 2013
-
- Sauvage J.-P. and Garpard P., From Non-Covalent Assemblies to Molecular Machines, Wiley, Weinheim, 2011
- Bruns C. J. and Stoddart J. F., The Nature of the Mechanical Bond: From Molecules to Machines, Wiley, New York, 2016
-
- Tachibana Y. Kihara N. Takata T. J. Am. Chem. Soc. 2004;126:3438–3439. doi: 10.1021/ja039461l. - DOI - PubMed
- Berna J. Alajarin M. Orenes R.-A. J. Am. Chem. Soc. 2010;132:10741–10747. doi: 10.1021/ja101151t. - DOI - PubMed
- Lewandowski B. De Bo G. Ward J. W. Papmeyer M. Kuschel S. Aldegunde M. J. Gramlich P. M. E. Heckmann D. Goldup S. M. D'Souza D. M. Fernandes A. E. Leigh D. A. Science. 2013;339:189–193. doi: 10.1126/science.1229753. - DOI - PubMed
- Lim J. Y. C. Yuntawattana N. Beer P. D. Williams C. K. Angew. Chem., Int. Ed. 2019;58:6007–6011. doi: 10.1002/anie.201901592. - DOI - PMC - PubMed
- Pairault N. Zhu H. Jansen D. Huber A. Daniliuc C. G. Grimme S. Niemeyer J. Angew. Chem., Int. Ed. 2020:59. doi: 10.1002/anie.201913781. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

