Dynamic pH responsivity of triazole-based self-immolative linkers
- PMID: 34094059
- PMCID: PMC8152797
- DOI: 10.1039/d0sc00532k
Dynamic pH responsivity of triazole-based self-immolative linkers
Abstract
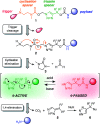
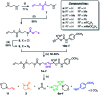
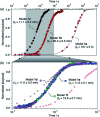
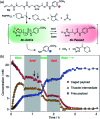
Gating the release of chemical payloads in response to transient signals is an important feature of 'smart' delivery systems. Herein, we report a triazole-based self-immolative linker that can be reversibly paused or slowed and restarted throughout its elimination cascade in response to pH changes in both organic and organic-aqueous solvents. The linker is conveniently prepared using the alkyne-azide cycloaddition reaction, which introduces a 1,4-triazole ring that expresses a pH-sensitive intermediate during its elimination sequence. Using a series of model compounds, we demonstrate that this intermediate can be switched between active and dormant states depending on the presence of acid or base, cleanly gating the release of payload in response to a fluctuating external stimulus.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Albert A. Nature. 1958;182:421–422. doi: 10.1038/182421a0. - DOI - PubMed
- Li J. Yu J. Zhao J. Wang J. Zheng S. Lin S. Chen L. Yang M. Jia S. Zhang X. Chen P. R. Nat. Chem. 2014;6:352–361. doi: 10.1038/nchem.1887. - DOI - PubMed
- Li Y. Liu G. Wang X. Hu J. Liu S. Angew. Chem., Int. Ed. 2016;55:1760–1764. doi: 10.1002/anie.201509401. - DOI - PubMed
- Ji X. Pan Z. Yu B. De La Cruz L. K. Zheng Y. Ke B. Wang B. Chem. Soc. Rev. 2019;48:1077–1094. doi: 10.1039/C8CS00395E. - DOI - PubMed
-
- Shao Q. Xing B. Chem. Soc. Rev. 2010;39:2835–2846. doi: 10.1039/B915574K. - DOI - PubMed
- Slanina T. Shrestha P. Palao E. Kand D. Peterson J. A. Dutton A. S. Rubinstein N. Weinstain R. Winter A. H. Klan P. J. Am. Chem. Soc. 2017;139:15168–15175. doi: 10.1021/jacs.7b08532. - DOI - PubMed
- Kand D. Pizarro L. Angel I. Avni A. Friedmann-Morvinski D. Weinstain R. Angew. Chem., Int. Ed. 2019;58:4659–4663. doi: 10.1002/anie.201900850. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

