Chemical synthesis of human syndecan-4 glycopeptide bearing O-, N-sulfation and multiple aspartic acids for probing impacts of the glycan chain and the core peptide on biological functions
- PMID: 34094105
- PMCID: PMC8159385
- DOI: 10.1039/d0sc01140a
Chemical synthesis of human syndecan-4 glycopeptide bearing O-, N-sulfation and multiple aspartic acids for probing impacts of the glycan chain and the core peptide on biological functions
Abstract
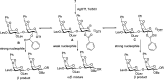
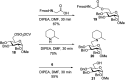
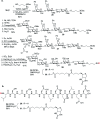
Proteoglycans are a family of complex glycoproteins with glycosaminoglycan chains such as heparan sulfate (HS) attached to the core protein backbone. Due to the high structural heterogeneity of HS in nature, it is challenging to decipher the respective roles of the HS chain and the core protein on proteoglycan functions. While the sulfation patterns of HS dictate many activities, the core protein can potentially impact HS functions. In order to decipher this, homogeneous proteoglycan glycopeptides are needed. Herein, we report the first successful synthesis of proteoglycan glycopeptides bearing multiple aspartic acids in the core peptide and O- and N-sulfations in the glycan chain, as exemplified by the syndecan-4 glycopeptides. To overcome the high acid sensitivities of sulfates and base sensitivities of the glycopeptide during synthesis, a new synthetic approach has been developed to produce a sulfated glycan chain on a peptide sequence prone to the formation of aspartimide side products. The availability of the structurally well-defined synthetic glycopeptide enabled the investigation of their biological functions including cytokine, growth factor binding and heparanase inhibition. Interestingly, the glycopeptide exhibited context dependent enhancement or decrease of biological activities compared to the peptide or the glycan alone. The results presented herein suggest that besides varying the sulfation patterns of HS, linking the HS chain to core proteins as in proteoglycans may be an additional approach to modulate biological functions of HS in nature.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
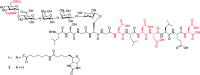



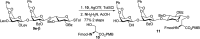




Similar articles
-
Sulfated Glycoprotein Synthesis.Methods Mol Biol. 2022;2530:1-17. doi: 10.1007/978-1-0716-2489-0_1. Methods Mol Biol. 2022. PMID: 35761038 Free PMC article.
-
Chemical synthesis of syndecan-3 glycopeptides bearing two heparan sulfate glycan chains.Angew Chem Int Ed Engl. 2014 Aug 18;53(34):9051-8. doi: 10.1002/anie.201404625. Epub 2014 Jun 30. Angew Chem Int Ed Engl. 2014. PMID: 24981920 Free PMC article.
-
Homoserine as an Aspartic Acid Precursor for Synthesis of Proteoglycan Glycopeptide Containing Aspartic Acid and a Sulfated Glycan Chain.J Org Chem. 2016 Dec 2;81(23):12052-12059. doi: 10.1021/acs.joc.6b02441. Epub 2016 Nov 10. J Org Chem. 2016. PMID: 27809505 Free PMC article.
-
Heparan Sulfate Biosynthesis and Sulfation Profiles as Modulators of Cancer Signalling and Progression.Front Oncol. 2021 Nov 11;11:778752. doi: 10.3389/fonc.2021.778752. eCollection 2021. Front Oncol. 2021. PMID: 34858858 Free PMC article. Review.
-
Investigation of the biological functions of heparan sulfate using a chemoenzymatic synthetic approach.RSC Chem Biol. 2021 Feb 22;2(3):702-712. doi: 10.1039/d0cb00199f. RSC Chem Biol. 2021. PMID: 34179782 Free PMC article. Review.
Cited by
-
Excavating proteoglycan structure-function relationships: modern approaches to capture the interactions of ancient biomolecules.Am J Physiol Cell Physiol. 2022 Aug 1;323(2):C415-C422. doi: 10.1152/ajpcell.00222.2022. Epub 2022 Jun 27. Am J Physiol Cell Physiol. 2022. PMID: 35759439 Free PMC article. Review.
-
Recent Advances in Enzymes and Chemoenzymatic Synthesis of Tetrasaccharide Linkage Region of Proteoglycans.Chembiochem. 2025 May 27;26(10):e202500095. doi: 10.1002/cbic.202500095. Epub 2025 May 16. Chembiochem. 2025. PMID: 40267247 Free PMC article. Review.
-
Convergent chemoenzymatic synthesis and biological evaluation of a heparan sulfate proteoglycan syndecan-1 mimetic.Chem Commun (Camb). 2021 Apr 7;57(27):3407-3410. doi: 10.1039/d1cc00796c. Epub 2021 Mar 9. Chem Commun (Camb). 2021. PMID: 33687395 Free PMC article.
-
Sulfated Glycoprotein Synthesis.Methods Mol Biol. 2022;2530:1-17. doi: 10.1007/978-1-0716-2489-0_1. Methods Mol Biol. 2022. PMID: 35761038 Free PMC article.
-
Structural Insights into Pixatimod (PG545) Inhibition of Heparanase, a Key Enzyme in Cancer and Viral Infections.Chemistry. 2022 Feb 19;28(11):e202104222. doi: 10.1002/chem.202104222. Epub 2022 Jan 31. Chemistry. 2022. PMID: 34981584 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

