In situ Raman study of the photoinduced behavior of dye molecules on TiO2(hkl) single crystal surfaces
- PMID: 34094107
- PMCID: PMC8159273
- DOI: 10.1039/d0sc00588f
In situ Raman study of the photoinduced behavior of dye molecules on TiO2(hkl) single crystal surfaces
Abstract
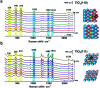
In dye-sensitized solar cells (DSSCs), the TiO2/dye interface significantly affects photovoltaic performance. However, the adsorption and photoinduced behavior of dye molecules on the TiO2 substrate remains unclear. Herein, shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) was used to study the adsorption and photoinduced behavior of dye (N719) molecules on different TiO2(hkl) surfaces. On TiO2(001) and TiO2(110) surfaces, the in situ SHINERS and mass spectrometry results indicate S[double bond, length as m-dash]C bond cleavage in the anchoring groups of adsorbed N719, whereas negligible bond cleavage occurs on the TiO2(111) surface. Furthermore, DFT calculations show the stability of the S[double bond, length as m-dash]C anchoring group on three TiO2(hkl) surfaces in the order TiO2(001) < TiO2(110) < TiO2(111), which correlated well with the observed photocatalytic activities. This work reveals the photoactivity of different TiO2(hkl) surface structures and can help with the rational design of DSSCs. Thus, this strategy can be applied to real-time probing of photoinduced processes on semiconductor single crystal surfaces.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- O'Regan B. Grätzel M. Nature. 1991;353:737. doi: 10.1038/353737a0. - DOI
- Parisi M. L. Maranghi S. Basosi R. Renewable Sustainable Energy Rev. 2014;39:124. doi: 10.1016/j.rser.2014.07.079. - DOI
- Fakharuddin A. Jose R. Brown T. M. Fabregat-Santiago F. Bisquert J. Energy Environ. Sci. 2014;7:3952. doi: 10.1039/C4EE01724B. - DOI
- Hagfeldt A. Boschloo G. Sun L. Kloo L. Pettersson H. Chem. Rev. 2010;110:6595. doi: 10.1021/cr900356p. - DOI - PubMed
-
- Green M. A. Hishikawa Y. Dunlop E. D. Levi D. H. Hohl-Ebinger J. Ho-Baillie A. W. Y. Prog. Photovoltaics Res. Appl. 2018;26:659. doi: 10.1002/pip.3035. - DOI
-
- Shakeel Ahmad M. Pandey A. K. Abd Rahim N. Renewable Sustainable Energy Rev. 2017;77:89. doi: 10.1016/j.rser.2017.03.129. - DOI
-
- Nazeeruddin M. K. Humphry-Baker R. Liska P. Grätzel M. J. Phys. Chem. B. 2003;107:8981. doi: 10.1021/jp022656f. - DOI
- Takeda N. Parkinson B. A. J. Am. Chem. Soc. 2003;125:5559. doi: 10.1021/ja0278483. - DOI - PubMed
- Zuo Q. Q. Feng Y. L. Chen S. Qiu Z. Xie L. Q. Xiao Z. Y. Yang Z. L. Mao B. W. Tian Z. Q. J. Phys. Chem. C. 2015;119:18396. doi: 10.1021/acs.jpcc.5b05318. - DOI
- Xie L. Q. Ding D. Zhang M. Chen S. Qiu Z. Yan J. W. Yang Z. L. Chen M. S. Mao B. W. Tian Z. Q. J. Phys. Chem. C. 2016;120:22500. doi: 10.1021/acs.jpcc.6b07763. - DOI
LinkOut - more resources
Full Text Sources

