Late-stage C(sp2)-H and C(sp3)-H glycosylation of C-aryl/alkyl glycopeptides: mechanistic insights and fluorescence labeling
- PMID: 34094117
- PMCID: PMC8152807
- DOI: 10.1039/d0sc01260b
Late-stage C(sp2)-H and C(sp3)-H glycosylation of C-aryl/alkyl glycopeptides: mechanistic insights and fluorescence labeling
Abstract
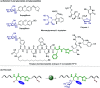
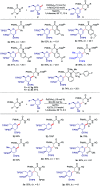
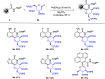
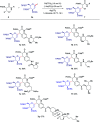
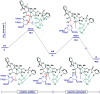
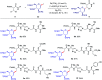
C(sp3)-H and C(sp2)-H glycosylations of structurally complex amino acids and peptides were accomplished through the assistance of triazole peptide-isosteres. The palladium-catalyzed peptide-saccharide conjugation provided modular access to structurally complex C-alkyl glycoamino acids, glycopeptides and C-aryl glycosides, while enabling the assembly of fluorescent-labeled glycoamino acids. The C-H activation approach represents an expedient and efficient strategy for peptide late-stage diversification in a programmable as well as chemo-, regio-, and diastereo-selective fashion.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- deGruyter J. N. Malins L. R. Baran P. S. Biochemistry. 2017;56:3863–3873. doi: 10.1021/acs.biochem.7b00536. - DOI - PMC - PubMed
- Krasnova L. Wong C.-H. Annu. Rev. Biochem. 2016;85:599–630. doi: 10.1146/annurev-biochem-060614-034420. - DOI - PubMed
- Danishefsky S. J. Allen J. R. Angew. Chem., Int. Ed. 2000;39:836–863. doi: 10.1002/(SICI)1521-3773(20000303)39:5<836::AID-ANIE836>3.0.CO;2-I. - DOI - PubMed
-
- Lepenies B. Seeberger P. H. Nat. Biotechnol. 2014;32:443–445. doi: 10.1038/nbt.2893. - DOI - PubMed
- Werdelin O. Meldal M. Jensen T. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9611–9613. doi: 10.1073/pnas.152345899. - DOI - PMC - PubMed
- Varki A. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. - DOI - PMC - PubMed
- Sears P. Wong C. H. Cell. Mol. Life Sci. 1998;54:223–252. doi: 10.1007/s000180050146. - DOI - PMC - PubMed
- Wong C.-H. J. Org. Chem. 2005;70:4219–4225. doi: 10.1021/jo050278f. - DOI - PubMed
-
- Payne R. J. Wong C.-H. Chem. Commun. 2010;46:21–43. doi: 10.1039/B913845E. - DOI - PubMed
- McKay M. J. Nguyen H. M. ACS Catal. 2012;2:1563–1595. doi: 10.1021/cs3002513. - DOI - PMC - PubMed
- Bonache M. A. Nuti F. Le Chevalier Isaad A. Real-Fernández F. Chelli M. Rovero P. Papini A. M. Tetrahedron Lett. 2009;50:4151–4153. doi: 10.1016/j.tetlet.2009.04.124. - DOI
- Pratt M. R. Bertozzi C. R. Chem. Soc. Rev. 2005;34:58–68. doi: 10.1039/B400593G. - DOI - PubMed
- Guo Z. Shao N. Med. Res. Rev. 2005;25:655–678. doi: 10.1002/med.20033. - DOI - PubMed
- Wang B. Liu Y. Jiao R. Feng Y. Li Q. Chen C. Liu L. He G. Chen G. J. Am. Chem. Soc. 2016;138:3926–3932. doi: 10.1021/jacs.6b01384. - DOI - PubMed
- Herzner H. Reipen T. Schultz M. Kunz H. Chem. Rev. 2000;100:4495–4538. doi: 10.1021/cr990308c. - DOI - PubMed
- Rodriguez J. O'Neill S. Walczak M. A. Nat. Prod. Rep. 2018;35:220–229. doi: 10.1039/C7NP00050B. - DOI - PubMed
- Gamblin D. P. Scanlan E. M. Davis B. G. Chem. Rev. 2009;109:131–163. doi: 10.1021/cr078291i. - DOI - PubMed
- Dai Y. Tian B. Chen H. Zhang Q. ACS Catal. 2019;9:2909–2915. doi: 10.1021/acscatal.9b00336. - DOI
LinkOut - more resources
Full Text Sources

