Programmable synthesis of multiply arylated cubanes through C-H metalation and arylation
- PMID: 34094145
- PMCID: PMC8159448
- DOI: 10.1039/d0sc01909g
Programmable synthesis of multiply arylated cubanes through C-H metalation and arylation
Abstract
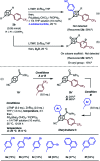
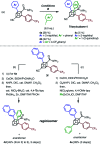
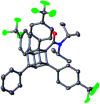
Cubane (C8H8), a cubic alkane, has long attracted attention owing to its unique 3D structure. In order to utilize the cubane scaffold widely in science and technology, a powerful method for synthesizing diverse cubane derivatives is required. Herein, we report the synthesis of mono-, di-, tri-, and tetra-arylated cubanes. Directed ortho-metalation with lithium base/alkyl zinc and subsequent palladium-catalyzed arylation enabled C-H metalation and arylation of cubane. This reaction allows the late-stage and regioselective installation of a wide range of aryl groups, realizing the first programmable synthesis of diverse multiply arylated cubanes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Palladium-Catalyzed Regioselective B(3,4,5,6)-H Tetra-Arylation of o-Carboranes.Inorg Chem. 2025 Jun 2;64(21):10612-10623. doi: 10.1021/acs.inorgchem.5c01257. Epub 2025 May 21. Inorg Chem. 2025. PMID: 40400108
-
Temperature-Controlled Selective Mono- vs. Di-ortho-Arylation for the Synthesis of Arylhydrazine Derivatives.Chemistry. 2022 Nov 25;28(66):e202202449. doi: 10.1002/chem.202202449. Epub 2022 Sep 26. Chemistry. 2022. PMID: 36043460
-
Palladium-catalyzed direct arylation of selenophene.J Org Chem. 2014 Jul 3;79(13):5987-92. doi: 10.1021/jo500094t. Epub 2014 Jun 16. J Org Chem. 2014. PMID: 24893620
-
From α-arylation of olefins to acylation with aldehydes: a journey in regiocontrol of the Heck reaction.Acc Chem Res. 2011 Aug 16;44(8):614-26. doi: 10.1021/ar200053d. Epub 2011 May 25. Acc Chem Res. 2011. PMID: 21612205 Review.
-
Recent Advances in Synthesis of Multiply Arylated/Alkylated Pyridines.Chem Rec. 2022 Sep;22(9):e202200099. doi: 10.1002/tcr.202200099. Epub 2022 Jun 14. Chem Rec. 2022. PMID: 35701177 Review.
Cited by
-
Substrate Plasticity Enables Group-Selective Transmetalation: Catalytic Stereospecific Cross-Couplings of Tertiary Boronic Esters.J Am Chem Soc. 2023 Sep 27;145(38):20755-20760. doi: 10.1021/jacs.3c07129. Epub 2023 Aug 31. J Am Chem Soc. 2023. PMID: 37651751 Free PMC article.
-
Synthesis of polysubstituted bicyclo[2.1.1]hexanes enabling access to new chemical space.Chem Sci. 2023 Aug 30;14(36):9885-9891. doi: 10.1039/d3sc03083k. eCollection 2023 Sep 20. Chem Sci. 2023. PMID: 37736652 Free PMC article.
-
Stereoselective polar radical crossover for the functionalization of strained-ring systems.Commun Chem. 2024 Jun 19;7(1):139. doi: 10.1038/s42004-024-01221-3. Commun Chem. 2024. PMID: 38898159 Free PMC article.
-
Three-dimensional saturated C(sp3)-rich bioisosteres for benzene.Nat Rev Chem. 2024 Aug;8(8):605-627. doi: 10.1038/s41570-024-00623-0. Epub 2024 Jul 9. Nat Rev Chem. 2024. PMID: 38982260 Free PMC article. Review.
-
Exploring Cuneanes as Potential Benzene Isosteres and Energetic Materials: Scope and Mechanistic Investigations into Regioselective Rearrangements from Cubanes.J Am Chem Soc. 2023 Aug 2;145(30):16355-16364. doi: 10.1021/jacs.3c03226. Epub 2023 Jul 24. J Am Chem Soc. 2023. PMID: 37486221 Free PMC article.
References
-
- Griffin G. W. Marchand A. P. Chem. Rev. 1989;89:997. doi: 10.1021/cr00095a003. - DOI
- Eaton P. E. Angew. Chem., Int. Ed. Engl. 1992;31:1421. doi: 10.1002/anie.199214211. - DOI
- Biegasiewicz K. F. Griffiths J. R. Savage G. P. Tsanaktsidis J. Priefer R. Chem. Rev. 2015;115:6719. doi: 10.1021/cr500523x. - DOI - PubMed
-
- Eaton P. E. Cole T. W. J. Am. Chem. Soc. 1964;86:962. doi: 10.1021/ja01059a072. - DOI
-
- DePuy C. H. Ponder B. W. Fitzpatrick J. D. J. Org. Chem. 1964;29:3508. doi: 10.1021/jo01035a014. - DOI
- Eaton P. E. Hudson R. A. J. Am. Chem. Soc. 1965;87:2769. doi: 10.1021/ja01090a052. - DOI
- Chapman N. B. Key J. M. Toyne K. J. J. Org. Chem. 1970;35:3860. doi: 10.1021/jo00836a062. - DOI
- Bliese M. Tsanaktsidis J. Aust. J. Chem. 1997;50:189. doi: 10.1071/C97021. - DOI
-
- Falkiner M. J. Littler S. W. McRae K. J. Savage G. P. Tsanaktsidis J. Org. Process Res. Dev. 2013;17:1503. doi: 10.1021/op400181g. - DOI
LinkOut - more resources
Full Text Sources

