Unusually high α-proton acidity of prolyl residues in cyclic peptides
- PMID: 34094148
- PMCID: PMC8159430
- DOI: 10.1039/d0sc02508a
Unusually high α-proton acidity of prolyl residues in cyclic peptides
Abstract
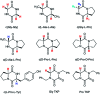
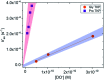
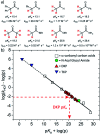
The acidity of the α-proton in peptides has an essential role in numerous biochemical reactions and underpins their stereochemical integrity, which is critical to their biological function. We report a detailed kinetic and computational study of the acidity of the α-proton in two cyclic peptide systems: diketopiperazine (DKP) and triketopiperazine (TKP). The kinetic acidity (protofugality) of the α-protons were determined though hydrogen deuterium exchange studies in aqueous solutions. The acidities of the α-proton in prolyl residues were increased by 3-89 fold relative to other amino acid residues (prolyl > glycyl ≫ alanyl > tyrosyl). Experimental and computational evidence for the stereoelectronic origins of this enhanced prolyl reactivity is presented. TKPs were 106-fold more reactive than their DKP analogues towards deprotonation, which we attribute to the advanced development of aromaticity in the earlier transition state for proton transfer in these cases. A Brønsted linear free energy analysis of the reaction data was conducted to provide estimates of α-proton pK as.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

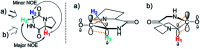

Similar articles
-
Philicities, Fugalities, and Equilibrium Constants.Acc Chem Res. 2016 May 17;49(5):952-65. doi: 10.1021/acs.accounts.6b00071. Epub 2016 Apr 25. Acc Chem Res. 2016. PMID: 27108991
-
Validation of the distal effect of electron-withdrawing groups on the stability of peptide enolates and its exploitation in the controlled stereochemical inversion of amino acid derivatives.J Org Chem. 2011 Aug 5;76(15):5907-14. doi: 10.1021/jo200994z. Epub 2011 Jul 7. J Org Chem. 2011. PMID: 21714508
-
Rationale for the acidity of Meldrum's acid. Consistent relation of C-H acidities to the properties of localized reactive orbital.J Org Chem. 2004 Jun 25;69(13):4309-16. doi: 10.1021/jo049456f. J Org Chem. 2004. PMID: 15202884
-
Acidity constants from DFT-based molecular dynamics simulations.J Phys Condens Matter. 2010 Jul 21;22(28):284116. doi: 10.1088/0953-8984/22/28/284116. Epub 2010 Jun 21. J Phys Condens Matter. 2010. PMID: 21399288 Review.
-
Brønsted acidity and the medium: fundamentals with a focus on ionic liquids.Chemphyschem. 2011 Jun 20;12(9):1622-32. doi: 10.1002/cphc.201100087. Epub 2011 Jun 1. Chemphyschem. 2011. PMID: 21633999 Review.
Cited by
-
Isolation and Identification of Cis-2,5-Diketopiperazine from a Novel Bacillus Strain and Synthesis of Its Four Stereoisomers.Mar Drugs. 2025 May 29;23(6):234. doi: 10.3390/md23060234. Mar Drugs. 2025. PMID: 40559643 Free PMC article.
-
Total Synthesis of (-)-Glionitrin A and B Enabled by an Asymmetric Oxidative Sulfenylation of Triketopiperazines.J Am Chem Soc. 2021 Dec 22;143(50):21218-21222. doi: 10.1021/jacs.1c10364. Epub 2021 Nov 22. J Am Chem Soc. 2021. PMID: 34808045 Free PMC article.
-
Abnormal (Hydroxy)proline Deuterium Content Redefines Hydrogen Chemical Mass.J Am Chem Soc. 2022 Feb 16;144(6):2484-2487. doi: 10.1021/jacs.1c12512. Epub 2022 Feb 2. J Am Chem Soc. 2022. PMID: 35107291 Free PMC article.
-
Synthesis of medium-ring lactams and macrocyclic peptide mimetics via conjugate addition/ring expansion cascade reactions.RSC Chem Biol. 2022 Feb 9;3(3):334-340. doi: 10.1039/d1cb00245g. eCollection 2022 Mar 9. RSC Chem Biol. 2022. PMID: 35359493 Free PMC article.
References
-
- Fischer G. Chem. Soc. Rev. 2000;29:119–127. doi: 10.1039/A803742F. - DOI
LinkOut - more resources
Full Text Sources

