Unusually high α-proton acidity of prolyl residues in cyclic peptides
- PMID: 34094148
- PMCID: PMC8159430
- DOI: 10.1039/d0sc02508a
Unusually high α-proton acidity of prolyl residues in cyclic peptides
Abstract
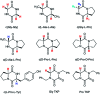
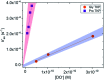
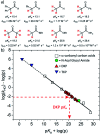
The acidity of the α-proton in peptides has an essential role in numerous biochemical reactions and underpins their stereochemical integrity, which is critical to their biological function. We report a detailed kinetic and computational study of the acidity of the α-proton in two cyclic peptide systems: diketopiperazine (DKP) and triketopiperazine (TKP). The kinetic acidity (protofugality) of the α-protons were determined though hydrogen deuterium exchange studies in aqueous solutions. The acidities of the α-proton in prolyl residues were increased by 3-89 fold relative to other amino acid residues (prolyl > glycyl ≫ alanyl > tyrosyl). Experimental and computational evidence for the stereoelectronic origins of this enhanced prolyl reactivity is presented. TKPs were 106-fold more reactive than their DKP analogues towards deprotonation, which we attribute to the advanced development of aromaticity in the earlier transition state for proton transfer in these cases. A Brønsted linear free energy analysis of the reaction data was conducted to provide estimates of α-proton pK as.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

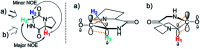

References
-
- Fischer G. Chem. Soc. Rev. 2000;29:119–127. doi: 10.1039/A803742F. - DOI
LinkOut - more resources
Full Text Sources

