Nickel-catalyzed three-component olefin reductive dicarbofunctionalization to access alkylborates
- PMID: 34094163
- PMCID: PMC8163243
- DOI: 10.1039/d0sc02054k
Nickel-catalyzed three-component olefin reductive dicarbofunctionalization to access alkylborates
Abstract
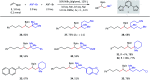
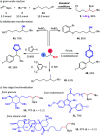
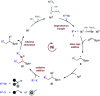
We report a three-component olefin reductive dicarbofunctionalization for constructing alkylborates, specifically, nickel-catalyzed reductive dialkylation and alkylarylation of vinyl boronates with a variety of alkyl bromides and aryl iodides. This reaction exhibits good coupling efficiency and excellent functional group compatibility, providing convenient access to the late-stage modification of complex natural products and drug molecules. Combined with alkylborate transformations, this reaction could also find applications in the modular and convergent synthesis of complex compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict of interest to report.
Figures




References
-
-
For selected reviews on olefin dicarbofunctionalization, see:
- Dhungana R. K. KC S. Basnet P. Giri R. Chem. Rec. 2018;18:1314. doi: 10.1002/tcr.201700098. - DOI - PubMed
- Zhang J.-S. Liu L. Chen T. Han L.-B. Chem.–Asian J. 2018;13:2277. doi: 10.1002/asia.201800647. - DOI - PubMed
- Ping Y. Li Y. Zhu J. Kong W. Angew. Chem., Int. Ed. 2019;58:1562. doi: 10.1002/anie.201806088. - DOI - PubMed
- Giri R. KC S. J. Org. Chem. 2018;83:3013. doi: 10.1021/acs.joc.7b03128. - DOI - PubMed
-
-
-
For selected examples on olefin dicarbofunctionalization, see:
- Urkalan K. B. Sigman M. S. Angew. Chem., Int. Ed. 2009;48:3146. doi: 10.1002/anie.200900218. - DOI - PMC - PubMed
- Trejos A. Fardost A. Yahiaoui S. Larhed M. Chem. Commun. 2009:7587. doi: 10.1039/B918358B. - DOI - PubMed
- McCammant M. S. Liao L. Sigman M. S. J. Am. Chem. Soc. 2013;135:4167. doi: 10.1021/ja3110544. - DOI - PMC - PubMed
- Liu Z. Zeng T. Yang K. S. Engle K. M. J. Am. Chem. Soc. 2016;138:15122. doi: 10.1021/jacs.6b09170. - DOI - PubMed
- Shrestha B. Basnet P. Dhungana R. K. Kc S. Thapa S. Sears J. M. Giri R. J. Am. Chem. Soc. 2017;139:10653. doi: 10.1021/jacs.7b06340. - DOI - PubMed
- Li W. Boon J. K. Zhao Y. Chem. Sci. 2018;9:600. doi: 10.1039/C7SC03149A. - DOI - PMC - PubMed
- Derosa J. Tran V. T. Boulous M. N. Chen J. S. Engle K. M. J. Am. Chem. Soc. 2017;139:10657. doi: 10.1021/jacs.7b06567. - DOI - PubMed
- Basnet P. Dhungana R. K. Thapa S. Shrestha B. Kc S. Sears J. M. Giri R. J. Am. Chem. Soc. 2018;140:7782. doi: 10.1021/jacs.8b03163. - DOI - PubMed
- Cong H. Fu G. C. J. Am. Chem. Soc. 2014;136:3788. doi: 10.1021/ja500706v. - DOI - PMC - PubMed
- You W. Brown M. K. J. Am. Chem. Soc. 2015;137:14578. doi: 10.1021/jacs.5b10176. - DOI - PubMed
- Walker J. A. Vickerman K. L. Humke J. N. Stanley L. M. J. Am. Chem. Soc. 2017;139:10228. doi: 10.1021/jacs.7b06191. - DOI - PubMed
- Qin T. Cornella J. Li C. Malins L. R. Edwards J. T. Kawamura S. Maxwell B. D. Eastgate M. D. Baran P. S. Science. 2016;352:801. doi: 10.1126/science.aaf6123. - DOI - PMC - PubMed
- Gu J.-W. Min Q.-Q. Yu L.-C. Zhang X. Angew. Chem., Int. Ed. 2016;55:12270. doi: 10.1002/anie.201606458. - DOI - PubMed
- Wu L. Wang F. Wan X. Wang D. Chen P. Liu G. J. Am. Chem. Soc. 2017;139:2904. doi: 10.1021/jacs.6b13299. - DOI - PubMed
- Watson M. P. Jacobsen E. N. J. Am. Chem. Soc. 2008;130:12594. doi: 10.1021/ja805094j. - DOI - PMC - PubMed
- You W. Brown M. K. J. Am. Chem. Soc. 2014;136:14730. doi: 10.1021/ja509056j. - DOI - PubMed
- Zhou L. Li S. Xu B. Ji D. Wu L. Liu Y. Zhang Z.-M. Zhang J. Angew. Chem., Int. Ed. 2020;59:2769. doi: 10.1002/anie.201913367. - DOI - PubMed
- Huang D. Olivieri D. Sun Y. Zhang P. Newhouse T. R. J. Am. Chem. Soc. 2019;141:16249. doi: 10.1021/jacs.9b09245. - DOI - PubMed
- Zhang Z.-M. Xu B. Wu L. Wu Y. Qian Y. Zhou L. Liu Y. Zhang J. Angew. Chem., Int. Ed. 2019;58:14653. doi: 10.1002/anie.201907840. - DOI - PubMed
- Koy M. Bellotti P. Katzenburg F. Daniliuc C. G. Glorius F. Angew. Chem., Int. Ed. 2020;59:2375. doi: 10.1002/anie.201911012. - DOI - PubMed
-
-
-
For selected examples on olefin reductive dicarbofunctionalization, see:
- Anthony D. Lin Q. Baudet J. Diao T. Angew. Chem., Int. Ed. 2019;58:3198. doi: 10.1002/anie.201900228. - DOI - PMC - PubMed
- Li J. Luo Y. Cheo H. W. Lan Y. Wu J. Chem. 2019;5:192. doi: 10.1016/j.chempr.2018.10.006. - DOI
- Wang K. Ding Z. Zhou Z. Kong W. J. Am. Chem. Soc. 2018;140:12364. doi: 10.1021/jacs.8b08190. - DOI - PubMed
- Yan C.-S. Peng Y. Xu X.-B. Wang Y.-W. Chem.–Eur. J. 2012;18:6039. doi: 10.1002/chem.201200190. - DOI - PubMed
- Tian Z.-X. Qiao J.-B. Xu G.-L. Pang X. Qi L. Ma W.-Y. Zhao Z.-Z. Duan J. Du Y.-F. Su P. Liu X.-Y. Shu X.-Z. J. Am. Chem. Soc. 2019;141:7637. doi: 10.1021/jacs.9b03863. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources

