Deoxygenative α-alkylation and α-arylation of 1,2-dicarbonyls
- PMID: 34094191
- PMCID: PMC8161533
- DOI: 10.1039/d0sc03118f
Deoxygenative α-alkylation and α-arylation of 1,2-dicarbonyls
Abstract
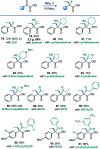
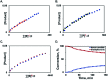
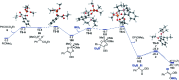
Construction of C-C bonds at the α-carbon is a challenging but synthetically indispensable approach to α-branched carbonyl motifs that are widely represented among drugs, natural products, and synthetic intermediates. Here, we describe a simple approach to generation of boron enolates in the absence of strong bases that allows for introduction of both α-alkyl and α-aryl groups in a reaction of readily accessible 1,2-dicarbonyls and organoboranes. Obviation of unselective, strongly basic and nucleophilic reagents permits carrying out the reaction in the presence of electrophiles that intercept the intermediate boron enolates, resulting in two new α-C-C bonds in a tricomponent process.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Singh M. S., Reactive Intermediates in Organic Chemistry: Structure, Mechanism, and Reactions, Wiley, Hoboken, 2014
- Shenvi R. A. O'Malley D. P. Baran P. S. Acc. Chem. Res. 2009;42:530–541. doi: 10.1021/ar800182r. - DOI - PMC - PubMed
- Afagh N. A. Yudin A. K. Angew. Chem., Int. Ed. 2010;49:262–310. doi: 10.1002/anie.200901317. - DOI - PubMed
-
- Zabicky J., The Chemistry of Metal Enolates; Patai Series: The Chemistry of Functional Groups, John Wiley & Sons, Chichester, UK, 2009
-
- Inomata K. Muraki M. Mukaiyama T. Bull. Chem. Soc. Jpn. 1973;46:1807–1810. doi: 10.1246/bcsj.46.1807. - DOI
-
- Kan S. B. J. Ng K. K. -H. Paterson I. Angew. Chem., Int. Ed. 2013;52:9097–9108. doi: 10.1002/anie.201303914. - DOI - PubMed
- Matsuo J. -i. Murakami M. Angew. Chem., Int. Ed. 2013;52:9109–9118. doi: 10.1002/anie.201303192. - DOI - PubMed
- He Z. Zajdlik A. Yudin A. K. Dalton Trans. 2014;43:11434–11451. doi: 10.1039/C4DT00817K. - DOI - PubMed
-
- Mukaiyama T. Murakami M. Oriyama T. Yamaguchi M. A. Chem. Lett. 1981;10:1193–1196. doi: 10.1246/cl.1981.1193. - DOI
- Matteson D. S. Moody R. J. Organometallics. 1982;1:20–28. doi: 10.1021/om00061a005. - DOI
- Iacono C. E. Stephens T. C. Rajan T. S. Pattison G. J. Am. Chem. Soc. 2018;140:2036–2040. doi: 10.1021/jacs.7b12941. - DOI - PubMed
- Sun W. Wang L. Xia C. Liu C. Angew. Chem., Int. Ed. 2018;57:5501–5505. doi: 10.1002/anie.201801679. - DOI - PubMed
- Lee B. Chirik P. J. J. Am. Chem. Soc. 2020;142:2429–2437. doi: 10.1021/jacs.9b11944. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

