Determination of the glycoprotein specificity of lectins on cell membranes through oxidative proteomics
- PMID: 34094216
- PMCID: PMC8162070
- DOI: 10.1039/d0sc04199h
Determination of the glycoprotein specificity of lectins on cell membranes through oxidative proteomics
Abstract
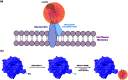
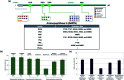
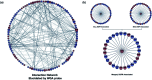
The cell membrane is composed of a network of glycoconjugates including glycoproteins and glycolipids that presents a dense matrix of carbohydrates playing critical roles in many biological processes. Lectin-based technology has been widely used to characterize glycoconjugates in tissues and cell lines. However, their specificity toward their putative glycan ligand and sensitivity in situ have been technologically difficult to study. Additionally, because they recognize primarily glycans, the underlying glycoprotein targets are generally not known. In this study, we employed lectin proximity oxidative labeling (Lectin PROXL) to identify cell surface glycoproteins that contain glycans that are recognized by lectins. Commonly used lectins were modified with a probe to produce hydroxide radicals in the proximity of the labeled lectins. The underlying polypeptides of the glycoproteins recognized by the lectins are oxidized and identified by the standard proteomic workflow. As a result, approximately 70% of identified glycoproteins were oxidized in situ by all the lectin probes, while only 5% of the total proteins were oxidized. The correlation between the glycosites and oxidation sites demonstrated the effectiveness of the lectin probes. The specificity and sensitivity of each lectin were determined using site-specific glycan information obtained through glycomic and glycoproteomic analyses. Notably, the sialic acid-binding lectins and the fucose-binding lectins had higher specificity and sensitivity compared to other lectins, while those that were specific to high mannose glycans have poor sensitivity and specificity. This method offers an unprecedented view of the interactions of lectins with specific glycoproteins as well as protein networks that are mediated by specific glycan types on cell membranes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts with this report.
Figures


References
-
- Paszek M. J. DuFort C. C. Rossier O. Bainer R. Mouw J. K. Godula K. Hudak J. E. Lakins J. N. Wijekoon A. C. Cassereau L. Rubashkin M. G. Magbanua M. J. Thorn K. S. Davidson M. W. Rugo H. S. Park J. W. Hammer D. A. Giannone G. Bertozzi C. R. Weaver V. M. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511(7509):319–325. doi: 10.1038/nature13535. - DOI - PMC - PubMed

