Surface hydration for antifouling and bio-adhesion
- PMID: 34094298
- PMCID: PMC8162394
- DOI: 10.1039/d0sc03690k
Surface hydration for antifouling and bio-adhesion
Abstract
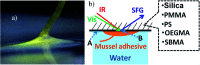
Antifouling properties of materials play crucial roles in many important applications such as biomedical implants, marine antifouling coatings, biosensing, and membranes for separation. Poly(ethylene glycol) (or PEG) containing polymers and zwitterionic polymers have been shown to be excellent antifouling materials. It is believed that their outstanding antifouling activity comes from their strong surface hydration. On the other hand, it is difficult to develop underwater glues, although adhesives with strong adhesion in a dry environment are widely available. This is related to dehydration, which is important for adhesion for many cases while water is the enemy of adhesion. In this research, we applied sum frequency generation (SFG) vibrational spectroscopy to investigate buried interfaces between mussel adhesive plaques and a variety of materials including antifouling polymers and control samples, supplemented by studies on marine animal (mussel) behavior and adhesion measurements. It was found that PEG containing polymers and zwitterionic polymers have very strong surface hydration in an aqueous environment, which is the key for their excellent antifouling performance. Because of the strong surface hydration, mussels do not settle on these surfaces even after binding to the surfaces with rubber bands. For control samples, SFG results indicate that their surface hydration is much weaker, and therefore mussels can generate adhesives to displace water to cause dehydration at the interface. Because of the dehydration, mussels can foul on the surfaces of these control materials. Our experiments also showed that if mussels were forced to deposit adhesives onto the PEG containing polymers and zwitterionic polymers, interfacial dehydration did not occur. However, even with the strong interfacial hydration, strong adhesion between mussel adhesives and antifouling polymer surfaces was detected, showing that under certain circumstances, interfacial water could enhance the interfacial bio-adhesion.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Dworak A. Utrata-Wesolek A. Otulakowski L. Trzebicka B. Polymers in Medicine – Direction of Development. Polymer. 2019;64:645–655.
LinkOut - more resources
Full Text Sources

