Base-catalyzed aryl halide isomerization enables the 4-selective substitution of 3-bromopyridines
- PMID: 34094310
- PMCID: PMC8162412
- DOI: 10.1039/d0sc02689a
Base-catalyzed aryl halide isomerization enables the 4-selective substitution of 3-bromopyridines
Abstract
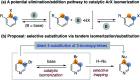
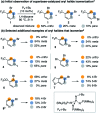
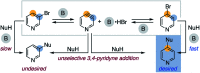
The base-catalyzed isomerization of simple aryl halides is presented and utilized to achieve the 4-selective etherification, hydroxylation and amination of 3-bromopyridines. Mechanistic studies support isomerization of 3-bromopyridines to 4-bromopyridines proceeds via pyridyne intermediates and that 4-substitution selectivity is driven by a facile aromatic substitution reaction. Useful features of a tandem aryl halide isomerization/selective interception approach to aromatic functionalization are demonstrated. Example benefits include the use of readily available and stable 3-bromopyridines in place of less available and stable 4-halogenated congeners and the ability to converge mixtures of 3- and 5-bromopyridines to a single 4-substituted product.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
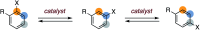


Similar articles
-
Pd/Norbornene: A Winning Combination for Selective Aromatic Functionalization via C-H Bond Activation.Acc Chem Res. 2016 Jul 19;49(7):1389-400. doi: 10.1021/acs.accounts.6b00165. Epub 2016 Jun 22. Acc Chem Res. 2016. PMID: 27333299
-
Copper-Catalyzed Electrophilic Etherification of Arylboronic Esters with Isoxazolidines.Chem Asian J. 2020 Jun 17;15(12):1869-1872. doi: 10.1002/asia.202000270. Epub 2020 May 25. Chem Asian J. 2020. PMID: 32352205
-
From α-arylation of olefins to acylation with aldehydes: a journey in regiocontrol of the Heck reaction.Acc Chem Res. 2011 Aug 16;44(8):614-26. doi: 10.1021/ar200053d. Epub 2011 May 25. Acc Chem Res. 2011. PMID: 21612205 Review.
-
One-pot borylation/amination reactions: syntheses of arylamine boronate esters from halogenated arenes.Org Lett. 2006 Mar 30;8(7):1407-10. doi: 10.1021/ol060205y. Org Lett. 2006. PMID: 16562903
-
Direct amination of aryl halides with ammonia.Chem Soc Rev. 2010 Nov;39(11):4130-45. doi: 10.1039/c003692g. Epub 2010 Sep 28. Chem Soc Rev. 2010. PMID: 20877862 Review.
Cited by
-
Nucleophilic C-H Etherification of Heteroarenes Enabled by Base-Catalyzed Halogen Transfer.J Am Chem Soc. 2021 Aug 18;143(32):12480-12486. doi: 10.1021/jacs.1c06481. Epub 2021 Aug 4. J Am Chem Soc. 2021. PMID: 34347457 Free PMC article.
-
Capturing Unstable Carbanionic Intermediates via Halogen Transfer: Base-Promoted Oxidative Coupling Reactions of α,α-Difluoromethylarenes.Angew Chem Int Ed Engl. 2025 May;64(21):e202502894. doi: 10.1002/anie.202502894. Epub 2025 Apr 14. Angew Chem Int Ed Engl. 2025. PMID: 40098196
References
-
-
For selected modes of reactivity, see:
- Terrier F., Modern Nucleophilic Aromatic Substitution, Wiley-VCH, Weinheim, 2013
- Magano J. Dunetz J. R. Chem. Rev. 2011;111:2177–2250. - PubMed
- Zhang N. Samanta S. R. Rosen B. M. Percec V. Chem. Rev. 2014;114:5848–5958. - PubMed
- Seyferth D. Organometallics. 2009;28:1598–1605.
- Twilton J. Le C. Zhang P. Shaw M. H. Evans R. W. MacMillan D. W. C. Nat. Rev. Chem. 2017;1:0052.
- Bunnett J. F. J. Chem. Educ. 1974;51:312–315.
-
-
-
For selected examples of acid-catalyzed rearrangement and disproportionation of haloarenes, see:
- Jacobs T. L. Winstein S. Ralls J. W. Robson J. H. J. Org. Chem. 1946;11:27–33. - PubMed
- Olah G. A. Tolgyesi W. S. Dear R. E. A. J. Org. Chem. 1962;27:3455–3464.
- Olah G. A. Meyer M. W. J. Org. Chem. 1962;27:3464–3469.
- Jacquesy J.-C. Jouannetaud M.-P. Tetrahedron Lett. 1982;23:1673–1676.
-
-
- Bunnett J. F. Acc. Chem. Res. 1972;5:139–147.
- Moyer Jr. C. E. Bunnett J. F. J. Am. Chem. Soc. 1963;85:1891–1893.
- Bunnett J. F. Scorrano G. J. Am. Chem. Soc. 1971;93:1190–1198.
-
- Bunnett J. F. Moyer Jr. C. E. J. Am. Chem. Soc. 1971;93:1183–1190.
- Bunnett J. F. Kearley Jr. F. J. J. Org. Chem. 1971;36:184–186.
LinkOut - more resources
Full Text Sources

