Organoborohydride-catalyzed Chichibabin-type C4-position alkylation of pyridines with alkenes assisted by organoboranes
- PMID: 34094401
- PMCID: PMC8162492
- DOI: 10.1039/d0sc04808a
Organoborohydride-catalyzed Chichibabin-type C4-position alkylation of pyridines with alkenes assisted by organoboranes
Abstract
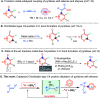
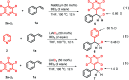
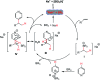
The first NaBEt3H-catalyzed intermolecular Chichibabin-type alkylation of pyridine and its derivatives with alkenes as the latent nucleophiles is presented with the assistance of BEt3, and a series of branched C4-alkylation pyridines, even highly congested all-carbon quaternary center-containing triarylmethanes can be obtained in a regiospecific manner. Therefore, the conventional reliance on high cost and low availability transition metal catalysts, prior formation of N-activated pyridines, organometallic reagents, and extra oxidation operation for the construction of a C-C bond at the C4-position of the pyridines in previous methods are not required. The corresponding mechanism and the key roles of the organoborane were elaborated by the combination of H/D scrambling experiments, 11B NMR studies, intermediate trapping experiments and computational studies. This straightforward and mechanistically distinct organocatalytic technology not only opens a new door for the classical but still far less well-developed Chichibabin-type reaction, but also sets up a new platform for the development of novel C-C bond-forming methods.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Daly J. W., Garraffo H. M. and Spande T. F., in Alkaloids: Chemical and Biological Perspectives, ed. W. W. Pelletier, Elsevier, New York, 1999, vol. 13, pp. 92–104
- Vitaku E. Smith D. T. Njardarson J. T. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. - DOI - PubMed
- Skrypink Y. G. Doroshenko T. F. Mater. Sci. 1996;32:537–544. doi: 10.1007/BF02539063. - DOI
- Leclerc N. Sanaur S. Galmiche L. Mathevet F. Attias A.-J. Fave J.-L. Roussel J. Hapiot P. Lemaître N. Geffroy B. Chem. Mater. 2005;17:502–513. doi: 10.1021/cm040358k. - DOI
-
-
For leading reviews, see:
- Snieckus V. Chem. Rev. 1990;90:879–933. doi: 10.1021/cr00104a001. - DOI
- Gros P. Fort Y. Eur. J. Org. Chem. 2002:3375–3383. doi: 10.1002/1099-0690(200210)2002:20<3375::AID-EJOC3375>3.0.CO;2-X. - DOI
- Schlosser M. Mongin F. Chem. Soc. Rev. 2007;36:1161–1172. doi: 10.1039/B706241A. - DOI - PubMed
- Gros P. C. Fort Y. Eur. J. Org. Chem. 2009:4199–4209. doi: 10.1002/ejoc.200900324. - DOI
- Ahamed M. Todd M. H. Eur. J. Org. Chem. 2010:5935–5942. doi: 10.1002/ejoc.201000877. - DOI
- Nakao Y. Synthesis. 2011:3209–3219. doi: 10.1055/s-0030-1260212. - DOI
- Zhuo C.-X. Zhang W. You S.-L. Angew. Chem., Int. Ed. 2012;51:12662–12686. doi: 10.1002/anie.201204822. - DOI - PubMed
- Bull J. A. Mousseau J. J. Pelletier G. Charette A. B. Chem. Rev. 2012;112:2642–2713. doi: 10.1021/cr200251d. - DOI - PubMed
- Ding Q. Zhou X. Fan R. Org. Biomol. Chem. 2014;12:4807–4815. doi: 10.1039/C4OB00371C. - DOI - PubMed
- Murakami K. Yamada S. Kaneda T. Itami K. Chem. Rev. 2017;117:9302–9332. doi: 10.1021/acs.chemrev.7b00021. - DOI - PubMed
-
-
-
For recent reviews on the Minisci reaction, see:
- Duncton M. A. J. MedChemComm. 2011;2:1135–1161. doi: 10.1039/C1MD00134E. - DOI
- Proctor R. S. J. Phipps R. J. Angew. Chem., Int. Ed. 2019;58:13666–13699. doi: 10.1002/anie.201900977. - DOI - PubMed
-
; For selected examples, see:
- Minisci F. Bernardi R. Bertini F. Galli R. Perchinummo M. Tetrahedron. 1971;27:3575–3579. doi: 10.1016/S0040-4020(01)97768-3. - DOI
- Fujiwara Y. Domingo V. Seiple I. B. Gianatassio R. Bel M. D. Baran P. S. J. Am. Chem. Soc. 2011;133:3292–3295. doi: 10.1021/ja111152z. - DOI - PMC - PubMed
- Molander G. A. Colombel V. Braz V. A. Org. Lett. 2011;13:1852–1855. doi: 10.1021/ol2003572. - DOI - PMC - PubMed
- Nagib D. A. MacMillan D. W. C. Nature. 2011;480:224–228. doi: 10.1038/nature10647. - DOI - PMC - PubMed
- Fujiwara Y. Dixon J. A. Hara F. O. Funder E. D. Dixon D. D. Rodriguez R. A. Baxter R. D. Herle B. Sach N. Collins M. R. Ishihara Y. Baran P. S. Nature. 2012;492:95–99. doi: 10.1038/nature11680. - DOI - PMC - PubMed
- Antonchick A. P. Burgmann L. Angew. Chem., Int. Ed. 2013;52:3267–3271. doi: 10.1002/anie.201209584. - DOI - PubMed
- Garza-Sanchez R. A. Tlahuext-Aca A. Tavakoli G. Glorious F. ACS Catal. 2017;7:4057–4061. doi: 10.1021/acscatal.7b01133. - DOI
- Cheng W.-M. Shang R. Fu Y. ACS Catal. 2017;7:907–911. doi: 10.1021/acscatal.6b03215. - DOI
- Proctor R. S. J. Davis H. J. Phipps R. J. Science. 2018;360:419–422. doi: 10.1126/science.aar6376. - DOI - PubMed
-
-
-
Selected literature of C2-selectivity, see:
- Jordan R. F. Taylor D. F. J. Am. Chem. Soc. 1989;111:778–779. doi: 10.1021/ja00184a081. - DOI
- Rodewald S. Jordan R. F. J. Am. Chem. Soc. 1994;116:4491–4492. doi: 10.1021/ja00089a054. - DOI
- Murakami M. Hori S. J. Am. Chem. Soc. 2003;125:4720–4721. doi: 10.1021/ja029829z. - DOI - PubMed
- Lewis J. C. Bergman R. G. Ellman J. A. J. Am. Chem. Soc. 2007;129:5332–5333. doi: 10.1021/ja070388z. - DOI - PMC - PubMed
- Nakao Y. Kanyiva K. S. Hiyama T. J. Am. Chem. Soc. 2008;130:2448–2449. doi: 10.1021/ja710766j. - DOI - PubMed
- Berman A. M. Lewis J. C. Bergman R. G. Ellman J. A. J. Am. Chem. Soc. 2008;130:14926–14927. doi: 10.1021/ja8059396. - DOI - PMC - PubMed
- Guan B.-T. Hou Z. J. Am. Chem. Soc. 2011;133:18086–18089. doi: 10.1021/ja208129t. - DOI - PubMed
- Kaneko H. Nagae H. Tsurugi H. Mashima K. J. Am. Chem. Soc. 2011;133:19626–19629. doi: 10.1021/ja208293h. - DOI - PubMed
- Liu B. Huang Y. Lan J. Song F. You J. Chem. Sci. 2013;4:2163–2167. doi: 10.1039/C3SC50348H. - DOI
- Johnson D. G. Lynam J. M. Mistry N. S. Slattery J. M. Thatcher R. J. Whitwood A. C. J. Am. Chem. Soc. 2013;135:2222–2234. doi: 10.1021/ja3097256. - DOI - PubMed
- Song G. Wylie W. N. O. Hou Z. J. Am. Chem. Soc. 2014;136:12209–12212. doi: 10.1021/ja504995f. - DOI - PubMed
- Nagae H. Tsurugi H. Mashima K. J. Am. Chem. Soc. 2015;137:640–643. doi: 10.1021/ja511964k. - DOI - PubMed
- Kundu A. Inoue M. Nagae H. Tsurugi H. Mashima K. J. Am. Chem. Soc. 2018;140:7332–7342. doi: 10.1021/jacs.8b03998. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

