Photoresponsive molecular tools for emerging applications of light in medicine
- PMID: 34094410
- PMCID: PMC8162950
- DOI: 10.1039/d0sc04187d
Photoresponsive molecular tools for emerging applications of light in medicine
Abstract
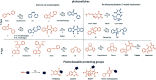
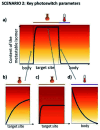
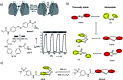
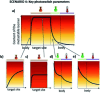
Light-based therapeutic and imaging modalities, which emerge in clinical applications, rely on molecular tools, such as photocleavable protecting groups and photoswitches that respond to photonic stimulus and translate it into a biological effect. However, optimisation of their key parameters (activation wavelength, band separation, fatigue resistance and half-life) is necessary to enable application in the medical field. In this perspective, we describe the applications scenarios that can be envisioned in clinical practice and then we use those scenarios to explain the necessary properties that the photoresponsive tools used to control biological function should possess, highlighted by examples from medical imaging, drug delivery and photopharmacology. We then present how the (photo)chemical parameters are currently being optimized and an outlook is given on pharmacological aspects (toxicity, solubility, and stability) of light-responsive molecules. With these interdisciplinary insights, we aim to inspire the future directions for the development of photocontrolled tools that will empower clinical applications of light.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures















Similar articles
-
Molecular photoswitches in aqueous environments.Chem Soc Rev. 2021 Nov 15;50(22):12377-12449. doi: 10.1039/d0cs00547a. Chem Soc Rev. 2021. PMID: 34590636 Free PMC article. Review.
-
Photopharmacology and photoresponsive drug delivery.Chem Soc Rev. 2025 Jun 16;54(12):5792-5835. doi: 10.1039/d5cs00125k. Chem Soc Rev. 2025. PMID: 40309857 Review.
-
Targeted Cancer Therapy Using Compounds Activated by Light.Cancers (Basel). 2021 Jun 29;13(13):3237. doi: 10.3390/cancers13133237. Cancers (Basel). 2021. PMID: 34209493 Free PMC article. Review.
-
Azoheteroarene and Diazocine Molecular Photoswitches: Self-Assembly, Responsive Materials and Photopharmacology.Angew Chem Int Ed Engl. 2023 Oct 16;62(42):e202304437. doi: 10.1002/anie.202304437. Epub 2023 Jul 3. Angew Chem Int Ed Engl. 2023. PMID: 37212536 Review.
-
Indigoid Photoswitches: Visible Light Responsive Molecular Tools.Acc Chem Res. 2018 May 15;51(5):1153-1163. doi: 10.1021/acs.accounts.7b00638. Epub 2018 Apr 25. Acc Chem Res. 2018. PMID: 29694014
Cited by
-
Photoswitchable and long-lived seven-membered cyclic singlet diradicals for the bioorthogonal photoclick reaction.Chem Sci. 2023 Nov 2;14(45):13254-13264. doi: 10.1039/d3sc03675h. eCollection 2023 Nov 22. Chem Sci. 2023. PMID: 38023496 Free PMC article.
-
Manipulation of Chemistry and Biology with Visible Light Using Tetra-ortho-Substituted Azobenzenes and Azonium Ions.Angew Chem Int Ed Engl. 2025 Jun 10;64(24):e202423506. doi: 10.1002/anie.202423506. Epub 2025 Apr 10. Angew Chem Int Ed Engl. 2025. PMID: 40152740 Free PMC article. Review.
-
Turning Red without Feeling Embarrassed─Xanthenium-Based Photocages for Red-Light-Activated Phototherapeutics.J Am Chem Soc. 2023 Feb 8;145(7):4026-34. doi: 10.1021/jacs.2c11499. Online ahead of print. J Am Chem Soc. 2023. PMID: 36752773 Free PMC article.
-
Recent Progress in Photoresponsive Biomaterials.Molecules. 2023 Apr 25;28(9):3712. doi: 10.3390/molecules28093712. Molecules. 2023. PMID: 37175122 Free PMC article. Review.
-
Synthesis and Study of Dibenzo[b, f]oxepine Combined with Fluoroazobenzenes-New Photoswitches for Application in Biological Systems.Molecules. 2022 Sep 8;27(18):5836. doi: 10.3390/molecules27185836. Molecules. 2022. PMID: 36144571 Free PMC article.
References
-
- Jia S. Fong W. K. Graham B. Boyd B. J. Chem. Mater. 2018;30:2873–2887. doi: 10.1021/acs.chemmater.8b00357. - DOI
-
- Molecular Switches, ed. B. L. Feringa and W. R. Browne, Wiley-VCH, Weinheim, 2nd edn, 2011
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

