Long periodic ripple in a 2D hybrid halide perovskite structure using branched organic spacers
- PMID: 34094428
- PMCID: PMC8162985
- DOI: 10.1039/d0sc04144k
Long periodic ripple in a 2D hybrid halide perovskite structure using branched organic spacers
Abstract
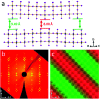
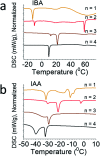
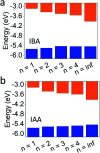
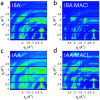
Two-dimensional (2D) halide perovskites have great promise in optoelectronic devices because of their stability and optical tunability, but the subtle effects on the inorganic layer when modifying the organic spacer remain unclear. Here, we introduce two homologous series of Ruddlesden-Popper (RP) structures using the branched isobutylammonium (IBA) and isoamylammonium (IAA) cations with the general formula (RA)2(MA) n-1Pb n I3n+1 (RA = IBA, IAA; MA = methylammonium n = 1-4). Surprisingly, the IAA n = 2 member results in the first modulated 2D perovskite structure with a ripple with a periodicity of 50.6 Å occurring in the inorganic slab diagonally to the [101] direction of the basic unit cell. This leads to an increase of Pb-I-Pb angles along the direction of the wave. Generally, both series show larger in-plane bond angles resulting from the additional bulkiness of the spacers compensating for the MA's small size. Larger bond angles have been shown to decrease the bandgap which is seen here with the bulkier IBA leading to both larger in-plane angles and lower bandgaps except for n = 2, in which the modulated structure has a lower bandgap because of its larger Pb-I-Pb angles. Photo-response was tested for the n = 4 compounds and confirmed, signaling their potential use in solar cell devices. We made films using an MACl additive which showed good crystallinity and preferred orientation according to grazing-incidence wide-angle scattering (GIWAXS). As exemplar, the two n = 4 samples were employed in devices with champion efficiencies of 8.22% and 7.32% for IBA and IAA, respectively.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Ball J. M. Lee M. M. Hey A. Snaith H. J. Energy Environ. Sci. 2013;6:1739–1743. doi: 10.1039/C3EE40810H. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

