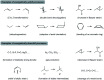
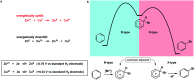
Synthetic half-reactions
- PMID: 34094448
- PMCID: PMC8162983
- DOI: 10.1039/d0sc03876h
Synthetic half-reactions
Abstract
This perspective on reactivity introduces Synthetic Half-Reactions (SHRs) as a way to analyze chemical transformations. SHRs denote either an uphill transformation leading to a higher energy state or a downhill transformation leading to a lower energy state. Using well-established processes, I show how the matching of different classes of SHRs offers a tool to classify chemical transformations. This raises the possibility to discover new processes by finding underappreciated combinations of endergonic and exergonic steps.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



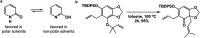
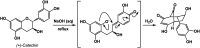


Similar articles
-
Energy- and atom-efficient chemical synthesis with endergonic photocatalysis.Nat Rev Chem. 2022 Oct;6(10):745-755. doi: 10.1038/s41570-022-00421-6. Epub 2022 Sep 23. Nat Rev Chem. 2022. PMID: 37117495 Review.
-
On the nature of organic and inorganic centers that bifurcate electrons, coupling exergonic and endergonic oxidation-reduction reactions.Chem Commun (Camb). 2018 Apr 19;54(33):4091-4099. doi: 10.1039/c8cc01530a. Chem Commun (Camb). 2018. PMID: 29624194
-
Force-Induced Catastrophes on Energy Landscapes: Mechanochemical Manipulation of Downhill and Uphill Bifurcations Explains the Ring-Opening Selectivity of Cyclopropanes.Chemphyschem. 2018 Apr 5;19(7):837-847. doi: 10.1002/cphc.201701209. Epub 2018 Feb 27. Chemphyschem. 2018. PMID: 29232496
-
Coupled reactions versus connected reactions: Coupling concepts with terms.Biochem Mol Biol Educ. 2007 Mar;35(2):85-8. doi: 10.1002/bmb.5. Biochem Mol Biol Educ. 2007. PMID: 21591066
-
A Decade of Electrochemical Dehydrogenative C,C-Coupling of Aryls.Acc Chem Res. 2020 Jan 21;53(1):45-61. doi: 10.1021/acs.accounts.9b00511. Epub 2019 Dec 18. Acc Chem Res. 2020. PMID: 31850730 Review.
Cited by
-
Iminologous epoxide ring-closure.Chem Sci. 2022 Oct 10;13(41):12175-12179. doi: 10.1039/d2sc04496j. eCollection 2022 Oct 26. Chem Sci. 2022. PMID: 36349099 Free PMC article.
-
Factors Controlling Diastereoselectivity and Reactivity in the Catalytic Aerobic Carbooxygenation of (E)-2-Fluoro-3-aryl-allyl Nitroacetates.Chem Asian J. 2025 Jul;20(13):e202500336. doi: 10.1002/asia.202500336. Epub 2025 May 2. Chem Asian J. 2025. PMID: 40318140 Free PMC article.
-
Pd-catalyzed Aerobic Cross-Dehydrogenative Coupling of Catechols with 2-Oxindoles and Benzofuranones: Reactivity Difference Between Monomer and Dimer.Chem Asian J. 2022 Oct 17;17(20):e202200807. doi: 10.1002/asia.202200807. Epub 2022 Sep 22. Chem Asian J. 2022. PMID: 36062560 Free PMC article.
-
Interrupted reactions in chemical synthesis.Nat Rev Chem. 2021 Sep;5(9):604-623. doi: 10.1038/s41570-021-00304-2. Epub 2021 Aug 4. Nat Rev Chem. 2021. PMID: 37118420 Review.
References
-
- King J. F. Rathore R. Lam J. Y. L. Guo Z. R. Klassen D. F. J. Am. Chem. Soc. 1992;114:3028. doi: 10.1021/ja00034a040. - DOI
-
- Mayr H. Patz M. Angew. Chem., Int. Ed. 1994;33:938. doi: 10.1002/anie.199409381. - DOI
-
- Houk K. N. Acc. Chem. Res. 1975;8:361. doi: 10.1021/ar50095a001. - DOI
-
- Bard A. J., Parsons R. and Jordan J., Standard Potentials in Aqueous Solution, CRC Press, 1985
-
This is to be contrasted with the decidedly non-mechanistic codification of construction reactions for systematic synthesis design:
- Hendrickson J. B. J. Am. Chem. Soc. 1975;97:5784. doi: 10.1021/ja00853a023. - DOI
Publication types
LinkOut - more resources
Full Text Sources

