On the electronic structure and hydrogen evolution reaction activity of platinum group metal-based high-entropy-alloy nanoparticles
- PMID: 34094468
- PMCID: PMC8163215
- DOI: 10.1039/d0sc02351e
On the electronic structure and hydrogen evolution reaction activity of platinum group metal-based high-entropy-alloy nanoparticles
Erratum in
-
Correction: On the electronic structure and hydrogen evolution reaction activity of platinum group metal-based high-entropy-alloy nanoparticles.Chem Sci. 2021 May 14;12(20):7196. doi: 10.1039/d1sc90104d. Chem Sci. 2021. PMID: 34123346 Free PMC article.
Abstract
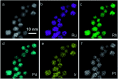
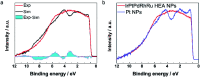
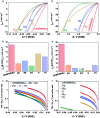
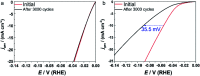
We report the synthesis of high-entropy-alloy (HEA) nanoparticles (NPs) consisting of five platinum group metals (Ru, Rh, Pd, Ir and Pt) through a facile one-pot polyol process. We investigated the electronic structure of HEA NPs using hard X-ray photoelectron spectroscopy, which is the first direct observation of the electronic structure of HEA NPs. Significantly, the HEA NPs possessed a broad valence band spectrum without any obvious peaks. This implies that the HEA NPs have random atomic configurations leading to a variety of local electronic structures. We examined the hydrogen evolution reaction (HER) and observed a remarkably high HER activity on HEA NPs. At an overpotential of 25 mV, the turnover frequencies of HEA NPs were 9.5 and 7.8 times higher than those of a commercial Pt catalyst in 0.05 M H2SO4 and 1.0 M KOH electrolytes, respectively. Moreover, the HEA NPs showed almost no loss during a cycling test and were much more stable than the commercial Pt catalyst. Our findings on HEA NPs may provide a new paradigm for the design of catalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Murty B., Yeh J. and Ranganthan S., High-Entropy Alloys, Elsevier, Netherlands, 2014
-
- George E. Raabe D. Ritchie R. Nat. Rev. Mater. 2019;4:515–534. doi: 10.1038/s41578-019-0121-4. - DOI
-
- Yeh J. Chen S. Gan J. Chin T. Shun T. Tsau C. Chang S. Metall. Mater. Trans. A. 2004;35:2533–2536. doi: 10.1007/s11661-006-0234-4. - DOI
-
- Malinovskis P. Fritze S. Riekehr L. Fieandt L. Cedervall J. Rehnlund D. Nyholm L. Lewin E. Jansson U. Mater. Des. 2018;149:51–62. doi: 10.1016/j.matdes.2018.03.068. - DOI
LinkOut - more resources
Full Text Sources

