Cross dehydrogenative C-O coupling catalysed by a catenane-coordinated copper(i)
- PMID: 34094485
- PMCID: PMC8163234
- DOI: 10.1039/d0sc05133k
Cross dehydrogenative C-O coupling catalysed by a catenane-coordinated copper(i)
Abstract
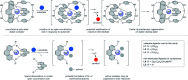
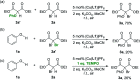
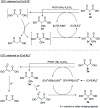
Catalytic activity of copper(i) complexes supported by phenanthroline-containing catenane ligands towards a new C(sp3)-O dehydrogenative cross-coupling of phenols and bromodicarbonyls is reported. As the phenanthrolines are interlocked by the strong and flexible mechanical bond in the catenane, the active catalyst with an open copper coordination site can be revealed only transiently and the stable, coordinatively saturated Cu(i) pre-catalyst is quickly regenerated after substrate transformation. Compared with a control Cu(i) complex supported by non-interlocked phenanthrolines, the catenane-supported Cu(i) is highly efficient with a broad substrate scope, and can be applied in gram-scale transformations without a significant loss of the catalytic activity. This work demonstrates the advantages of the catenane ligands that provide a dynamic and responsive copper coordination sphere, highlighting the potential of the mechanical bond as a design element in transition metal catalyst development.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
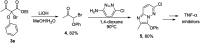
References
-
-
For selected reviews, see:
- Evano G. and Blanchard N.Copper Mediated Cross-Coupling Reactions, John Wiley & Sons Inc., New Jersey, 2013
- Beletskaya I. P. Cheprakov A. V. Coord. Chem. Rev. 2004;248:2337. doi: 10.1016/j.ccr.2004.09.014. - DOI
- Evano G. Blanchard N. Toumi M. Chem. Rev. 2008;108:3054. doi: 10.1021/cr8002505. - DOI - PubMed
- Prokopcova H. Kappe C. O. Angew. Chem., Int. Ed. 2008;47:3674. doi: 10.1002/anie.200800449. - DOI - PubMed
- Surry D. S. Buchwald S. L. Chem. Sci. 2010;1:13. doi: 10.1039/C0SC00107D. - DOI - PMC - PubMed
- Evano G. Theunissen C. Pradal A. Nat. Prod. Rep. 2013;30:1467. doi: 10.1039/C3NP70071B. - DOI - PubMed
- Casitas A. Ribas X. Chem. Sci. 2013;4:2301. doi: 10.1039/C3SC21818J. - DOI
- Bhunia S. Pawar G. G. Kumar S. V. Jiang Y. Ma D. Angew. Chem., Int. Ed. 2017;56:16136. doi: 10.1002/anie.201701690. - DOI - PubMed
- Hossain A. Bhattacharyya A. Reiser O. Science. 2019;364:eaav9713. doi: 10.1126/science.aav9713. - DOI - PubMed
-
-
-
For examples of phenanthroline-supported, coordinatively saturated copper-based coupling catalysts:
- Hossain A. Engl S. Lutsker E. Reiser O. ACS Catal. 2019;9:1103. doi: 10.1021/acscatal.8b04188. - DOI
- Rawner T. Lutsker E. Kaiser C. A. Reiser O. ACS Catal. 2018;8:3950. doi: 10.1021/acscatal.8b00847. - DOI
-
-
- Jones G. R. Anastasaki A. Whitfield R. Engelis N. Liarou E. Haddleton D. M. Angew. Chem., Int. Ed. 2018;57:10468. doi: 10.1002/anie.201802091. - DOI - PubMed
- Wendlandt A. E. Suess A. M. Stahl S. S. Angew. Chem., Int. Ed. 2011;50:11062. doi: 10.1002/anie.201103945. - DOI - PubMed
- Trammell R. Rajabimoghadam K. Garcia-Bosch I. Chem. Rev. 2019;119:2954. doi: 10.1021/acs.chemrev.8b00368. - DOI - PMC - PubMed
- Iwamatsu S. Matsubara K. Nagashima H. J. Org. Chem. 1999;64:9625. doi: 10.1021/jo9912146. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

