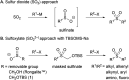
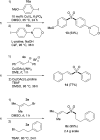
Silyloxymethanesulfinate as a sulfoxylate equivalent for the modular synthesis of sulfones and sulfonyl derivatives
- PMID: 34094489
- PMCID: PMC8163199
- DOI: 10.1039/d0sc02947e
Silyloxymethanesulfinate as a sulfoxylate equivalent for the modular synthesis of sulfones and sulfonyl derivatives
Abstract
An efficient protocol for the modular synthesis of sulfones and sulfonyl derivatives has been developed utilizing sodium tert-butyldimethylsilyloxymethanesulfinate (TBSOMS-Na) as a sulfoxylate (SO2 2-) equivalent. TBSOMS-Na, easily prepared from the commercial reagents Rongalite™ and TBSCl, serves as a potent nucleophile in S-alkylation and Cu-catalyzed S-arylation reactions with alkyl and aryl electrophiles. The sulfone products thus obtained can undergo the second bond formation at the sulfur center with various electrophiles without a separate unmasking step to afford sulfones and sulfonyl derivatives such as sulfonamides and sulfonyl fluorides.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
D.-K. Kim, H.-S. Um, H. Park, and C. Lee are inventors on patent application 10-2019-0126427 (Republic of Korea) submitted by Seoul National University that covers the modular synthesis of sulfones and sulfonyl derivatives using TBSOMS-Na.
Figures
References
-
-
For reviews on the construction of C–S bonds, see:
- Kondo T. Mitsudo T.-A. Chem. Rev. 2000;100:3205. doi: 10.1021/cr9902749. - DOI - PubMed
- Liu N.-W. Liang S. Manolikakes G. Synthesis. 2016;48:1939. doi: 10.1055/s-0035-1560444. - DOI
- Zhu J. Yang W.-C. Wang X.-D. Wu L. Adv. Synth. Catal. 2018;360:386. doi: 10.1002/adsc.201701194. - DOI
-
. For reviews on the utility of sulfonyl motifs, see:
- El-Hibri M. J. and Weinberg S. A., Encyclopedia of Polymer Science and Technology, Jonn Wiley & Sons, New York, 2002, vol. 4, pp. 1–26
- Dizman C. Tasdelen M. A. Yagci Y. Polym. Int. 2013;62:991. doi: 10.1002/pi.4525. - DOI
- Feng M. Tang B. Liang S. H. Jiang X. Curr. Top. Med. Chem. 2016;16:1200. doi: 10.2174/1568026615666150915111741. - DOI - PMC - PubMed
- Devendar P. Yang G.-F. Top. Curr. Chem. 2017;375:82. doi: 10.1007/s41061-017-0169-9. - DOI - PubMed
- Scott K. A. Njardarson J. T. Top. Curr. Chem. 2018;376:5. doi: 10.1007/s41061-018-0184-5. - DOI - PubMed
- Trost B. M. Kalnmals C. A. Chem.–Eur. J. 2019;25:11193. doi: 10.1002/chem.201984862. - DOI - PubMed
-
-
-
For a seminal report, see:
- Woolven H. González-Rodríguez C. Marco I. Thomson A. L. Willis M. C. Org. Lett. 2011;13:4876. doi: 10.1021/ol201957n. - DOI - PubMed
-
. For reviews on DABSO, see:
- Emmett E. J. Willis M. C. Asian J. Org. Chem. 2015;4:602. doi: 10.1002/ajoc.201500103. - DOI
- Willis M. C. Phosphorus, Sulfur Silicon Relat. Elem. 2019;194:654. doi: 10.1080/10426507.2019.1602623. - DOI - PMC - PubMed
-
LinkOut - more resources
Full Text Sources