Direct, stereoselective thioglycosylation enabled by an organophotoredox radical strategy
- PMID: 34094490
- PMCID: PMC8163235
- DOI: 10.1039/d0sc04136j
Direct, stereoselective thioglycosylation enabled by an organophotoredox radical strategy
Abstract
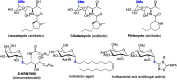
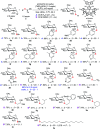
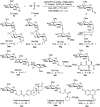
While strategies involving a 2e- transfer pathway have dictated glycosylation development, the direct glycosylation of readily accessible glycosyl donors as radical precursors is particularly appealing because of high radical anomeric selectivity and atom- and step-economy. However, the development of the radical process has been challenging owing to notorious competing reduction, elimination and/or SN side reactions of commonly used, labile glycosyl donors. Here we introduce an organophotocatalytic strategy through which glycosyl bromides can be efficiently converted into corresponding anomeric radicals by photoredox mediated HAT catalysis without a transition metal or a directing group and achieve highly anomeric selectivity. The power of this platform has been demonstrated by the mild reaction conditions enabling the synthesis of challenging α-1,2-cis-thioglycosides, the tolerance of various functional groups and the broad substrate scope for both common pentoses and hexoses. Furthermore, this general approach is compatible with both sp2 and sp3 sulfur electrophiles and late-stage glycodiversification for a total of 50 substrates probed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Rudd P. M. Elliot T. Cresswell P. Wilson I. A. Dwek R. A. Science. 2001;291:2370. doi: 10.1126/science.291.5512.2370. - DOI - PubMed
- Davis B. G. Chem. Rev. 2002;102:579. doi: 10.1021/cr0004310. - DOI - PubMed
- Doores K. J. Gamblin D. P. Davis B. G. Chem.–Eur. J. 2006;12:656. doi: 10.1002/chem.200500557. - DOI - PubMed
- Lowe J. B. Cell. 2001;104:809. doi: 10.1016/S0092-8674(01)00277-X. - DOI - PubMed
-
- Thayer D. A. Yu H. N. Galan M. C. Wong C.-H. Angew. Chem., Int. Ed. 2005;44:4596. doi: 10.1002/anie.200500090. - DOI - PubMed
- Pachamuthu K. Schmidt R. R. Chem. Rev. 2006;106:160. doi: 10.1021/cr040660c. - DOI - PubMed
- Rye C. S. Withers S. G. Carbohydr. Res. 2004;339:699. doi: 10.1016/j.carres.2003.12.011. - DOI - PubMed
- Amso Z. Bisset S. W. yang S.-H. Harris P. W. R. Wright T. H. Navo C. D. Patchett M. L. Norris G. E. Brimble M. A. Chem. Sci. 2018;9:1686. doi: 10.1039/C7SC04383J. - DOI - PMC - PubMed
-
- Schwarz S. Shen J. Kadlec K. Wang Y. Michael G. B. Feßler A. T. Vester B. Cold Spring Harb. Perspect. Med. 2016;6:a027037. doi: 10.1101/cshperspect.a027037. - DOI - PMC - PubMed
- Oman T. J. Boettcher J. M. Wang H. Okalibe X. N. van der Donk W. A. Nat. Chem. Biol. 2011;7:78. doi: 10.1038/nchembio.509. - DOI - PMC - PubMed
- Hsieh Y. S. Y. Wilkinson B. L. O'Connell M. R. Mackay J. P. Matthews J. M. Payne R. J. Org. Lett. 2012;14:1910. doi: 10.1021/ol300557g. - DOI - PubMed
- Biswas S. Garcia De Gonzalo C. V. Repka L. M. van der Donk W. A. ACS Chem. Biol. 2017;12:2965. doi: 10.1021/acschembio.7b00819. - DOI - PMC - PubMed
-
- Comber R. N. Friedrich J. D. Dunshee D. A. Petty S. L. Secrist J. A. Carbohydr. Res. 1994;262:245. doi: 10.1016/0008-6215(94)84182-9. - DOI - PubMed
- Mangte D. V. Deshmukh S. P. Heteroat. Chem. 2007;18:390. doi: 10.1002/hc.20310. - DOI
- El-Sayed W. A. Fathi N. M. Gad W. A. El-Ashry E. S. H. J. Carbohyd. Chem. 2008;27:357. doi: 10.1080/07328300802262778. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

