Investigating the Existence of Ribosomal Protein L5 Gene in Syrian Strain of Leishmania tropica Genome: Sequencing It and Evaluating Its Immune Response as DNA Vaccine
- PMID: 34094593
- PMCID: PMC8163552
- DOI: 10.1155/2021/6617270
Investigating the Existence of Ribosomal Protein L5 Gene in Syrian Strain of Leishmania tropica Genome: Sequencing It and Evaluating Its Immune Response as DNA Vaccine
Abstract
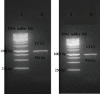
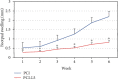
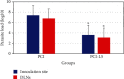
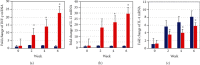
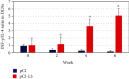
Cutaneous leishmaniasis in Syria is caused mainly by Leishmania tropica. It represents a serious health problem, which has aggravated further after the civil war in the country. Until now, there are no effective protective strategies, safe therapy, or efficacious vaccine to protect from this infection. DNA vaccines represent a promising approach for achieving protection against leishmaniasis. The L5 ribosomal protein plays fundamental roles in the assembly process of the ribosome subunits, so this study has chosen the ribosomal protein L5 gene to design a DNA vaccine against Leishmania tropica infection. After proving the existence of the ribosomal protein L5 gene in a Syrian strain of Leishmania tropica (LCED Syrian 01), it was sequenced and cloned into a pCI plasmid, and the designed DNA vaccine was administered to BALB/c mice. The protective response was evaluated by measuring lesion development in immunized BALB/c mice for 6 weeks after challenging mice with the parasite. RT-qPCR was used to quantify IL-12, IFN-γ, and IL-4 in draining lymph nodes (DLNs) of immunized mice. In the final week, the parasite burden was determined in footpad lesions and local draining lymph nodes (DLNs). This study demonstrated the presence and expression of the ribosomal protein L5 gene in the Syrian strain of Leishmania tropica promastigotes. The sequence of the ribosomal protein cDNA L5 gene was determined and published in Genbank. The gene size was 918 bp. Expression was also demonstrated at the level of cDNA. This study also demonstrated that vaccination with the ribosomal protein L5 gene induces TH1 response in immunized mice. This response prevents the partial development of a skin lesion of Leishmania.
Copyright © 2021 Mohammad Maarouf and Alyaa A. Abdlwahab.
Conflict of interest statement
There is no conflict of interest.
Figures



Similar articles
-
Evaluation of Immune Response and Protection Induced by V-ATPase Subunit F as DNA Vaccine Against Leishmania tropica (LCED Syrian 01) After Detection and Sequencing.Avicenna J Med Biotechnol. 2020 Jan-Mar;12(1):9-16. Avicenna J Med Biotechnol. 2020. PMID: 32153733 Free PMC article.
-
Cross-protective efficacy of Leishmania infantum LiHyD protein against tegumentary leishmaniasis caused by Leishmania major and Leishmania braziliensis species.Acta Trop. 2016 Jun;158:220-230. doi: 10.1016/j.actatropica.2016.03.011. Epub 2016 Mar 11. Acta Trop. 2016. PMID: 26976272
-
Vaccination with Leishmania infantum acidic ribosomal P0 but not with nucleosomal histones proteins controls Leishmania infantum infection in hamsters.PLoS Negl Trop Dis. 2015 Feb 2;9(2):e0003490. doi: 10.1371/journal.pntd.0003490. eCollection 2015 Feb. PLoS Negl Trop Dis. 2015. PMID: 25642946 Free PMC article.
-
Leishmania tropica: suggestive evidences for the effect of infectious dose on pathogenicity and immunogenicity in an experimental model.Parasitol Res. 2018 Sep;117(9):2949-2956. doi: 10.1007/s00436-018-5991-7. Epub 2018 Jul 5. Parasitol Res. 2018. PMID: 29978420
-
Detection, genotyping, and phylogenetic analysis of Leishmania isolates collected from infected Jordanian residents and Syrian refugees who suffered from cutaneous leishmaniasis.Parasitol Res. 2019 Mar;118(3):793-805. doi: 10.1007/s00436-019-06222-z. Epub 2019 Feb 7. Parasitol Res. 2019. PMID: 30729301
Cited by
-
Leishmania tarentolae as Potential Live Vaccine Co-Expressing Distinct Salivary Gland Proteins Against Experimental Cutaneous Leishmaniasis in BALB/c Mice Model.Front Immunol. 2022 Jun 10;13:895234. doi: 10.3389/fimmu.2022.895234. eCollection 2022. Front Immunol. 2022. PMID: 35757692 Free PMC article.
References
-
- Taheri T., Rafati S. Leishmaniasis: recombinant DNA vaccination and different approaches for vaccine development. Clinical Investigation. 2013;3(11):1023–1044. doi: 10.4155/cli.13.99. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

