Minimalist de novo Design of Protein Catalysts
- PMID: 34094654
- PMCID: PMC8174531
- DOI: 10.1021/acscatal.9b02509
Minimalist de novo Design of Protein Catalysts
Abstract
The field of protein design has grown enormously in the past few decades. In this review we discuss the minimalist approach to design of artificial enzymes, in which protein sequences are created with the minimum number of elements for folding and function. This method relies on identifying starting points in catalytically inert scaffolds for active site installation. The progress of the field from the original helical assemblies of the 1980s to the more complex structures of the present day is discussed, highlighting the variety of catalytic reactions which have been achieved using these methods. We outline the strengths and weaknesses of the minimalist approaches, describe representative design cases and put it in the general context of the de novo design of proteins.
Keywords: Protein design; catalysis; design approaches; enzymology; minimalism.
Figures



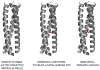
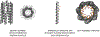
Similar articles
-
De Novo Design of Four-Helix Bundle Metalloproteins: One Scaffold, Diverse Reactivities.Acc Chem Res. 2019 May 21;52(5):1148-1159. doi: 10.1021/acs.accounts.8b00674. Epub 2019 Apr 11. Acc Chem Res. 2019. PMID: 30973707 Free PMC article.
-
Minimalist Design of Allosterically Regulated Protein Catalysts.Methods Enzymol. 2016;580:191-202. doi: 10.1016/bs.mie.2016.05.055. Epub 2016 Jun 29. Methods Enzymol. 2016. PMID: 27586334
-
Catalysis and Electron Transfer in De Novo Designed Metalloproteins.Chem Rev. 2022 Jul 27;122(14):12046-12109. doi: 10.1021/acs.chemrev.1c01025. Epub 2022 Jun 28. Chem Rev. 2022. PMID: 35763791 Free PMC article. Review.
-
De novo protein design, a retrospective.Q Rev Biophys. 2020 Feb 11;53:e3. doi: 10.1017/S0033583519000131. Q Rev Biophys. 2020. PMID: 32041676 Free PMC article. Review.
-
Design of Artificial Enzymes: Insights into Protein Scaffolds.Chembiochem. 2023 Mar 14;24(6):e202200566. doi: 10.1002/cbic.202200566. Epub 2023 Jan 3. Chembiochem. 2023. PMID: 36418221 Review.
Cited by
-
Supramolecular Peptide Nanostructures Regulate Catalytic Efficiency and Selectivity.Angew Chem Int Ed Engl. 2023 Jun 26;62(26):e202303755. doi: 10.1002/anie.202303755. Epub 2023 May 17. Angew Chem Int Ed Engl. 2023. PMID: 37194941 Free PMC article.
-
Synergistic Interactions Are Prevalent in Catalytic Amyloids.Chembiochem. 2020 Sep 14;21(18):2611-2614. doi: 10.1002/cbic.202000205. Epub 2020 Jun 9. Chembiochem. 2020. PMID: 32329215 Free PMC article.
-
Extension of an Atom-Atom Dispersion Function to Halogen Bonds and Its Use for Rational Design of Drugs and Biocatalysts.J Phys Chem A. 2021 Mar 4;125(8):1787-1799. doi: 10.1021/acs.jpca.0c11347. Epub 2021 Feb 23. J Phys Chem A. 2021. PMID: 33620223 Free PMC article.
-
Single amino acid bionanozyme for environmental remediation.Nat Commun. 2022 Mar 21;13(1):1505. doi: 10.1038/s41467-022-28942-0. Nat Commun. 2022. PMID: 35314678 Free PMC article.
-
Biocatalysts Based on Peptide and Peptide Conjugate Nanostructures.Biomacromolecules. 2021 May 10;22(5):1835-1855. doi: 10.1021/acs.biomac.1c00240. Epub 2021 Apr 12. Biomacromolecules. 2021. PMID: 33843196 Free PMC article. Review.
References
-
- Kiss G; Celebi-Ölcum N; Moretti R; Baker D; Houk KN, Computational Enzyme Design. Angew. Chem. Int. Ed , 2013, 52, 5700–5725. - PubMed
-
- Kries H; Blomberg R; Hilvert D, De Novo Enzymes by Computational Design. Curr. Opin. Chem. Biol, 2013, 17, 221–228. - PubMed
-
- Peacock AFA, Recent Advances in Designed Coiled Coils and Helical Bundles with Inorganic Prosthetic Groups - from Structural to Functional Applications. Curr. Opin. Chem. Biol, 2016, 31, 160–165. - PubMed
-
- Fruton JS, Contrasts in Scientific Style. Emil Fischer and Franz Hofmeister: Their Research Groups and Their Theory of Protein Structure. Proc. Am. Philos. Soc, 1985, 129, 313–370. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
