Downregulation of HBx Restrains Proliferation, Migration, and Invasion of HepG2 Cells
- PMID: 34094815
- PMCID: PMC8140855
- DOI: 10.1155/2021/6615979
Downregulation of HBx Restrains Proliferation, Migration, and Invasion of HepG2 Cells
Abstract
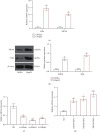
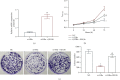
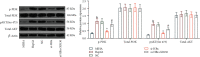
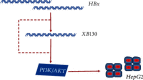
Liver cancer is a major contributor to cancer-related death with poor survival for sufferers. Meanwhile, Hepatic B virus X protein (HBx) and XB130 are likely to participate in the pathogenesis of liver cancer. However, the detailed mechanism of HBx/XB130 in liver cancer remains to be further investigated. Our study explored the effects of HBx/XB130 on liver cancer progression. HBx and XB130 expression was detected by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and Western blot. Overexpression of HBx and XB130 was found in liver cancer tissues and cells. Mechanistic study revealed that HBx could bind to and positively regulate XB130 in HepG2 cells. Subsequently, HBx expression was knocked down, while XB130 was overexpressed in HepG2 cells in order to observe the specific role of HBx/XB130 in liver cancer in vitro. Results of CCK-8, Transwell, wound healing, and colony formation assays suggested that HBx could mediate biological function of HepG2 cells by activating the XB130-mediated PI3K/AKT pathway. In summary, our data illustrate that inhibition of HBx effectively suppressed proliferation and metastasis and induced apoptosis of liver cancer cells, which might be partially reversed by XB130. HBx and XB130 may be potential targets for liver cancer pathogenesis.
Copyright © 2021 Chaoqun Huang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




Similar articles
-
Linc00152 promotes cancer progression in hepatitis B virus-associated hepatocellular carcinoma.Biomed Pharmacother. 2017 Jun;90:100-108. doi: 10.1016/j.biopha.2017.03.031. Epub 2017 Mar 24. Biomed Pharmacother. 2017. PMID: 28343069
-
HBx mediated Increase of SIRT1 Contributes to HBV-related Hepatocellular Carcinoma Tumorigenesis.Int J Med Sci. 2020 Jul 9;17(12):1783-1794. doi: 10.7150/ijms.43491. eCollection 2020. Int J Med Sci. 2020. PMID: 32714081 Free PMC article.
-
Hepatitis B virus X protein promotes human hepatoma cell growth via upregulation of transcription factor AP2α and sphingosine kinase 1.Acta Pharmacol Sin. 2015 Oct;36(10):1228-36. doi: 10.1038/aps.2015.38. Epub 2015 Jun 15. Acta Pharmacol Sin. 2015. PMID: 26073327 Free PMC article.
-
Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma.World J Gastroenterol. 2014 Aug 14;20(30):10238-48. doi: 10.3748/wjg.v20.i30.10238. World J Gastroenterol. 2014. PMID: 25132741 Free PMC article. Review.
-
The Epigenetic Modulation of Cancer and Immune Pathways in Hepatitis B Virus-Associated Hepatocellular Carcinoma: The Influence of HBx and miRNA Dysregulation.Front Immunol. 2021 Apr 29;12:661204. doi: 10.3389/fimmu.2021.661204. eCollection 2021. Front Immunol. 2021. PMID: 33995383 Free PMC article. Review.
Cited by
-
The First Yarrowia lipolytica Yeast Models Expressing Hepatitis B Virus X Protein: Changes in Mitochondrial Morphology and Functions.Microorganisms. 2022 Sep 10;10(9):1817. doi: 10.3390/microorganisms10091817. Microorganisms. 2022. PMID: 36144419 Free PMC article.
-
Transcriptional co-activators: emerging roles in signaling pathways and potential therapeutic targets for diseases.Signal Transduct Target Ther. 2023 Nov 13;8(1):427. doi: 10.1038/s41392-023-01651-w. Signal Transduct Target Ther. 2023. PMID: 37953273 Free PMC article. Review.
-
Glucose metabolism reprogramming promotes immune escape of hepatocellular carcinoma cells.Explor Target Antitumor Ther. 2023;4(3):519-536. doi: 10.37349/etat.2023.00149. Epub 2023 Jun 30. Explor Target Antitumor Ther. 2023. PMID: 37455832 Free PMC article. Review.
-
The pathogenesis of liver cancer and the therapeutic potential of bioactive substances.Front Pharmacol. 2022 Oct 5;13:1029601. doi: 10.3389/fphar.2022.1029601. eCollection 2022. Front Pharmacol. 2022. PMID: 36278230 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

