CAR-T cells: Early successes in blood cancer and challenges in solid tumors
- PMID: 34094824
- PMCID: PMC8144892
- DOI: 10.1016/j.apsb.2020.10.020
CAR-T cells: Early successes in blood cancer and challenges in solid tumors
Abstract
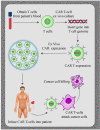
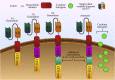
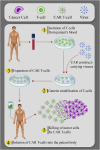
New approaches to cancer immunotherapy have been developed, showing the ability to harness the immune system to treat and eliminate cancer. For many solid tumors, therapy with checkpoint inhibitors has shown promise. For hematologic malignancies, adoptive and engineered cell therapies are being widely developed, using cells such as T lymphocytes, as well as natural killer (NK) cells, dendritic cells, and potentially others. Among these adoptive cell therapies, the most active and advanced therapy involves chimeric antigen receptor (CAR)-T cells, which are T cells in which a chimeric antigen receptor is used to redirect specificity and allow T cell recognition, activation and killing of cancers, such as leukemia and lymphoma. Two autologous CAR-T products have been approved by several health authorities, starting with the U.S. Food and Drug Administration (FDA) in 2017. These products have shown powerful, inducing, long-lasting effects against B cell cancers in many cases. In distinction to the results seen in hematologic malignancies, the field of using CAR-T products against solid tumors is in its infancy. Targeting solid tumors and trafficking CAR-T cells into an immunosuppressive microenvironment are both significant challenges. The goal of this review is to summarize some of the most recent aspects of CAR-T cell design and manufacturing that have led to successes in hematological malignancies, allowing the reader to appreciate the barriers that must be overcome to extend CAR-T therapies to solid tumors successfully.
Keywords: Chimeric antigen receptor (CAR); Genetic engineering; Immunotherapy; T cell therapy.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
Stephan A. Grupp receives study support from 10.13039/100004336Novartis, Kite, 10.13039/501100011725Servier, and Vertex. He consults for Novartis, Roche, GSK, Humanigen, CBMG, and Janssen/JnJ. He is on study steering committees or scientific advisory boards for Novartis, Allogene, Jazz, Adaptimmune, TCR2, Cellectis, Juno, and Vertex/CRISPR. He has a patent (Toxicity management for anti-tumor activity of CARs, WO 2014011984 A1) that is managed according to the University of Pennsylvania patent policy. The other authors declare no conflicts of interests in this work.
Figures
References
-
- Imai K., Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Canc. 2006;6:714–727. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

