Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies
- PMID: 34094826
- PMCID: PMC8144894
- DOI: 10.1016/j.apsb.2021.03.013
Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies
Abstract
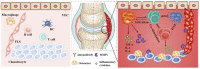
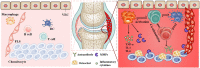
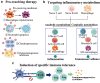
Increasing understanding of the pathogenesis of rheumatoid arthritis (RA) has remarkably promoted the development of effective therapeutic regimens of RA. Nevertheless, the inadequate response to current therapies in a proportion of patients, the systemic toxicity accompanied by long-term administration or distribution in non-targeted sites and the comprised efficacy caused by undesirable bioavailability, are still unsettled problems lying across the full remission of RA. So far, these existing limitations have inspired comprehensive academic researches on nanomedicines for RA treatment. A variety of versatile nanocarriers with controllable physicochemical properties, tailorable drug release pattern or active targeting ability were fabricated to enhance the drug delivery efficiency in RA treatment. This review aims to provide an up-to-date progress regarding to RA treatment using nanomedicines in the last 5 years and concisely discuss the potential application of several newly emerged therapeutic strategies such as inducing the antigen-specific tolerance, pro-resolving therapy or regulating the immunometabolism for RA treatments.
Keywords: Immune tolerance; Inflammation resolution; Liposome; Micelle; Nanomedicines; Rheumatoid arthritis; Stimulus-responsive delivery systems; Targeted drug delivery.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
All authors declare no competing interests.
Figures

References
-
- McInnes I.B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337. - PubMed
-
- Li Z.G. A new look at rheumatology in China—opportunities and challenges. Nat Rev Rheumatol. 2015;11:313–317. - PubMed
-
- Smolen J.S., Aletaha D., Barton A., Burmester G.R., Emery P., Firestein G.S. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. - PubMed
-
- Kim K., Bang S.Y., Lee H.S., Bae S.C. Update on the genetic architecture of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:13–24. - PubMed
-
- Weyand C.M., Fujii H., Shao L., Goronzy J.J. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–588. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

