Development of an Aerobic Glycolysis Index for Predicting the Sorafenib Sensitivity and Prognosis of Hepatocellular Carcinoma
- PMID: 34094917
- PMCID: PMC8169983
- DOI: 10.3389/fonc.2021.637971
Development of an Aerobic Glycolysis Index for Predicting the Sorafenib Sensitivity and Prognosis of Hepatocellular Carcinoma
Abstract
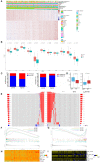
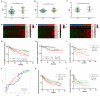
Hepatocellular carcinoma (HCC) is a deadly tumor with high heterogeneity. Aerobic glycolysis is a common indicator of tumor growth and plays a key role in tumorigenesis. Heterogeneity in distinct metabolic pathways can be used to stratify HCC into clinically relevant subgroups, but these have not yet been well-established. In this study, we constructed a model called aerobic glycolysis index (AGI) as a marker of aerobic glycolysis using genomic data of hepatocellular carcinoma from The Cancer Genome Atlas (TCGA) project. Our results showed that this parameter inferred enhanced aerobic glycolysis activity in tumor tissues. Furthermore, high AGI is associated with poor tumor differentiation and advanced stages and could predict poor prognosis including reduced overall survival and disease-free survival. More importantly, the AGI could accurately predict tumor sensitivity to Sorafenib therapy. Therefore, the AGI may be a promising biomarker that can accurately stratify patients and improve their treatment efficacy.
Keywords: Sorafenib; aerobic glycolysis; biomarker; hepatocellar carcinoma; prognosis.
Copyright © 2021 Pan, Hu, Jiang, Xia, Maher, Lin, Mao, Zhao, Cai, Xu, Xu and Cai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis.J Exp Clin Cancer Res. 2020 Jan 30;39(1):24. doi: 10.1186/s13046-020-1528-x. J Exp Clin Cancer Res. 2020. PMID: 32000827 Free PMC article.
-
RRAD inhibits aerobic glycolysis, invasion, and migration and is associated with poor prognosis in hepatocellular carcinoma.Tumour Biol. 2016 Apr;37(4):5097-105. doi: 10.1007/s13277-015-4329-7. Epub 2015 Nov 6. Tumour Biol. 2016. PMID: 26546438
-
USP29-mediated HIF1α stabilization is associated with Sorafenib resistance of hepatocellular carcinoma cells by upregulating glycolysis.Oncogenesis. 2021 Jul 16;10(7):52. doi: 10.1038/s41389-021-00338-7. Oncogenesis. 2021. PMID: 34272356 Free PMC article.
-
Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma.J Exp Clin Cancer Res. 2020 Jul 6;39(1):126. doi: 10.1186/s13046-020-01629-4. J Exp Clin Cancer Res. 2020. PMID: 32631382 Free PMC article. Review.
-
Glucometabolic Reprogramming in the Hepatocellular Carcinoma Microenvironment: Cause and Effect.Cancer Manag Res. 2020 Jul 17;12:5957-5974. doi: 10.2147/CMAR.S258196. eCollection 2020. Cancer Manag Res. 2020. PMID: 32765096 Free PMC article. Review.
Cited by
-
Emerging trends and knowledge networks in pan-cancer sorafenib resistance: a 20-year bibliometric investigation.Front Pharmacol. 2025 Jul 23;16:1581820. doi: 10.3389/fphar.2025.1581820. eCollection 2025. Front Pharmacol. 2025. PMID: 40771926 Free PMC article.
References
LinkOut - more resources
Full Text Sources

