A Phase 2 Randomised Clinical Trial Assessing the Tolerability of Two Different Ratios of Medicinal Cannabis in Patients With High Grade Gliomas
- PMID: 34094937
- PMCID: PMC8176855
- DOI: 10.3389/fonc.2021.649555
A Phase 2 Randomised Clinical Trial Assessing the Tolerability of Two Different Ratios of Medicinal Cannabis in Patients With High Grade Gliomas
Abstract
Background: Cannabis for cancer is very topical and, given the use of illicit cannabis preparations used in this vulnerable population, research investigating standardised, quality-assured medicinal cannabis is critical to inform clinicians and assist patient safety.
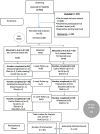
Methods: A randomized trial involving adult patients diagnosed with a high-grade glioma, no history of substance abuse, liver or kidney damage or myocardial infarction were eligible for inclusion in a tolerability study on two different ratios of medicinal cannabis. Baseline screening of brain morphology, blood pathology, functional status, and cognition was conducted. A retrospective control group was used for comparison for secondary outcomes.
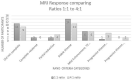
Results: Participants (n=88) were on average 53.3 years old. A paired t-test assessed the Functional Assessment of Cancer Therapy for Brain Cancer (FACT-Br) between groups from baseline to week 12 found that the 1:1 ratio favoured both physical (p=0.025) and functional (p=0.014) capacity and improved sleep (p=0.009). Analysis of changes from baseline to week 12 also found 11% of 61 participants had a reduction in disease, 34% were stable, 16% had slight enhancement, and 10% had progressive disease. No serious adverse events occurred. Side effects included dry mouth, tiredness at night, dizziness, drowsiness.
Conclusion: This study demonstrated that a single nightly dose of THC-containing medicinal cannabis was safe, had no serious adverse effects and was well tolerated in patients. Medicinal cannabis significantly improved sleep, functional wellbeing, and quality of life.
Clinical trial registration: Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373556&isReview=true, identifier ACTRN12617001287325.
Keywords: cannabis (marijuana); glioblastoma multiforme (GBM); high-grade glioma (HGG); medicinal marijuana; quality of life; tolerability.
Copyright © 2021 Schloss, Lacey, Sinclair, Steel, Sughrue, Sibbritt and Teo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A Quality-of-Life Evaluation Study Assessing Health-Related Quality of Life in Patients Receiving Medicinal Cannabis (the QUEST Initiative): Protocol for a Longitudinal Observational Study.JMIR Res Protoc. 2021 Nov 24;10(11):e32327. doi: 10.2196/32327. JMIR Res Protoc. 2021. PMID: 34821570 Free PMC article.
-
Universal cannabis outcomes from the Climate and Preventure (CAP) study: a cluster randomised controlled trial.Subst Abuse Treat Prev Policy. 2018 Sep 25;13(1):34. doi: 10.1186/s13011-018-0171-4. Subst Abuse Treat Prev Policy. 2018. PMID: 30253790 Free PMC article. Clinical Trial.
-
Pharmacokinetics, Safety, and Tolerability of a Medicinal Cannabis Formulation in Patients with Chronic Non-cancer Pain on Long-Term High Dose Opioid Analgesia: A Pilot Study.Pain Ther. 2022 Mar;11(1):171-189. doi: 10.1007/s40122-021-00344-y. Epub 2021 Dec 18. Pain Ther. 2022. PMID: 34921662 Free PMC article.
-
Efficacy, tolerability and safety of cannabis-based medicines for cancer pain : A systematic review with meta-analysis of randomised controlled trials.Schmerz. 2019 Oct;33(5):424-436. doi: 10.1007/s00482-019-0373-3. Schmerz. 2019. PMID: 31073761 English.
-
Cannabis-based medicines--GW pharmaceuticals: high CBD, high THC, medicinal cannabis--GW pharmaceuticals, THC:CBD.Drugs R D. 2003;4(5):306-9. doi: 10.2165/00126839-200304050-00005. Drugs R D. 2003. PMID: 12952500 Review.
Cited by
-
Anticancer effect of minor phytocannabinoids in preclinical models of multiple myeloma.Biofactors. 2024 Nov-Dec;50(6):1208-1219. doi: 10.1002/biof.2078. Epub 2024 May 17. Biofactors. 2024. PMID: 38760945 Free PMC article.
-
Palliative care and end-of-life care in adults with malignant brain tumors.Neuro Oncol. 2023 Mar 14;25(3):447-456. doi: 10.1093/neuonc/noac216. Neuro Oncol. 2023. PMID: 36271873 Free PMC article.
-
Adverse events associated with the use of cannabis-based products in people living with cancer: a systematic scoping review.Support Care Cancer. 2024 Dec 18;33(1):40. doi: 10.1007/s00520-024-09087-w. Support Care Cancer. 2024. PMID: 39694905 Free PMC article.
-
The Effects of a Combination of Medical Cannabis, Melatonin, and Oxygen-Ozone Therapy on Glioblastoma Multiforme: A Case Report.Reports (MDPI). 2023 May 5;6(2):22. doi: 10.3390/reports6020022. Reports (MDPI). 2023. PMID: 40729189 Free PMC article.
-
Endocannabinoid signaling in glioma.Glia. 2023 Jan;71(1):127-138. doi: 10.1002/glia.24173. Epub 2022 Mar 24. Glia. 2023. PMID: 35322459 Free PMC article.
References
-
- National Academies of Sciences, Engineering and Medicine . The Health Effects of Cannabis and Cannabinoids. In: The Current State of Evidence and Recommendations for Research. Washington DC: The National Academies Press; (2017). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources