Von Willebrand Factor Facilitates Intravascular Dissemination of Microsporidia Encephalitozoon hellem
- PMID: 34095003
- PMCID: PMC8176104
- DOI: 10.3389/fcimb.2021.694957
Von Willebrand Factor Facilitates Intravascular Dissemination of Microsporidia Encephalitozoon hellem
Abstract
Microsporidia are a group of spore-forming, fungus-related pathogens that can infect both invertebrates and vertebrates including humans. The primary infection site is usually digestive tract, but systemic infections occur as well and cause damages to organs such as lung, brain, and liver. The systemic spread of microsporidia may be intravascular, requiring attachment and colonization in the presence of shear stress. Von Willebrand Factor (VWF) is a large multimeric intravascular protein and the key attachment sites for platelets and coagulation factors. Here in this study, we investigated the interactions between VWF and microsporidia Encephalitozoon hellem (E. hellem), and the modulating effects on E. hellem after VWF binding. Microfluidic assays showed that E. hellem binds to ultra-large VWF strings under shear stress. In vitro germination assay and infection assay proved that E. hellem significantly increased the rates of germination and infection, and these effects would be reversed by VWF blocking antibody. Mass spectrometry analysis further revealed that VWF-incubation altered various aspects of E. hellem including metabolic activity, levels of structural molecules, and protein maturation. Our findings demonstrated that VWF can bind microsporidia in circulation, and modulate its pathogenicity, including promoting germination and infection rate. VWF facilitates microsporidia intravascular spreading and systemic infection.
Keywords: Encephalitozoon hellem; infection; intravascular dissemination; microsporidia; von Willebrand factor.
Copyright © 2021 Bao, Mo, An, Luo, Poncz, Pan, Li and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
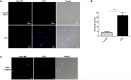



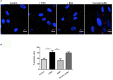

References
-
- Barber B. E., William T., Grigg M. J., Parameswaran U., Piera K. A., Price R. N., et al. (2015). Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria. PloS Pathog. 11, e1004558. 10.1371/journal.ppat.1004558 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

