Triazole-Modified Peptidomimetics: An Opportunity for Drug Discovery and Development
- PMID: 34095086
- PMCID: PMC8172596
- DOI: 10.3389/fchem.2021.674705
Triazole-Modified Peptidomimetics: An Opportunity for Drug Discovery and Development
Abstract
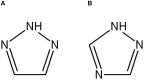
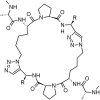
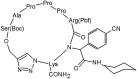
Peptidomimetics play a fundamental role in drug design due to their preferential properties regarding natural peptides. In particular, compounds possessing nitrogen-containing heterocycles have been intensively studied in recent years. The triazolyl moiety incorporation decreases the molecule susceptibility to enzymatic degradation, reduction, hydrolysis, and oxidation. In fact, peptides containing triazole rings are a typical example of peptidomimetics. They have all the advantages over classic peptides. Both efficient synthetic methods and biological activity make these systems an interesting and promising object of research. Peptide triazole derivatives display a diversity of biological properties and can be obtained via numerous synthetic strategies. In this review, we have highlighted the importance of the triazole-modified peptidomimetics in the field of drug design. We present an overview on new achievements in triazolyl-containing peptidomimetics synthesis and their biological activity as inhibitors of enzymes or against cancer, viruses, bacteria, or fungi. The relevance of above-mentioned compounds was confirmed by their comparison with unmodified peptides.
Keywords: 1,2,3-triazole; 1,2,4-triazole; CuAAC; antibacterial triazoles; antifungal triazoles; antiviral triazoles; disulphide bond mimetic; enzyme inhibitors.
Copyright © 2021 Staśkiewicz, Ledwoń, Rovero, Papini and Latajka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
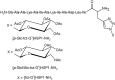
- Cantel S., Le Chevalier Isaad A., Scrima M., Levy J. J., DiMarchi R. D., Rovero P., et al. (2008). Synthesis and conformational analysis of a cyclic peptide obtained via i to i+4 intramolecular side-chain to side-chain azide–alkyne 1,3-dipolar cycloaddition. J. Org. Chem. 73, 5663–5674. 10.1021/jo800142s - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

