Boosting Anion Transport Activity of Diamidocarbazoles by Electron Withdrawing Substituents
- PMID: 34095089
- PMCID: PMC8172623
- DOI: 10.3389/fchem.2021.690035
Boosting Anion Transport Activity of Diamidocarbazoles by Electron Withdrawing Substituents
Abstract
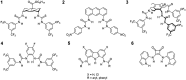
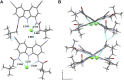
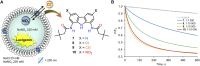
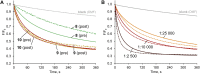
Artificial chloride transporters have been intensely investigated in view of their potential medicinal applications. Recently, we have established 1,8-diamidocarbazoles as a versatile platform for the development of active chloride carriers. In the present contribution, we investigate the influence of various electron-withdrawing substituents in positions 3 and 6 of the carbazole core on the chloride transport activity of these anionophores. Using lucigenin assay and large unilamellar vesicles as models, the 3,6-dicyano- and 3,6-dinitro- substituted receptors were found to be highly active and perfectly deliverable chloride transporters, with EC50,270s value as low as 22 nM for the Cl-/NO3 - exchange. Mechanistic studies revealed that diamidocarbazoles form 1:1 complexes with chloride in lipid bilayers and facilitate chloride/nitrate exchange by carrier mechanism. Furthermore, owing to its increased acidity, the 3,6-dinitro- substituted receptor acts as a pH-switchable transporter, with physiologically relevant apparent pKa of 6.4.
Keywords: LUVs; anion transport; carbazole; chloride transport; liposomes; lucigenin; pH-switchable transport.
Copyright © 2021 Maslowska-Jarzyna, Korczak and Chmielewski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
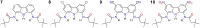


References
LinkOut - more resources
Full Text Sources

