The Intestinal Epithelium - Fluid Fate and Rigid Structure From Crypt Bottom to Villus Tip
- PMID: 34095127
- PMCID: PMC8172987
- DOI: 10.3389/fcell.2021.661931
The Intestinal Epithelium - Fluid Fate and Rigid Structure From Crypt Bottom to Villus Tip
Abstract
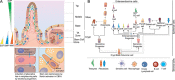
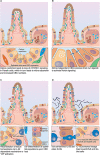
The single-layered, simple epithelium of the gastro-intestinal tract controls nutrient uptake, coordinates our metabolism and shields us from pathogens. Despite its seemingly simple architecture, the intestinal lining consists of highly distinct cell populations that are continuously renewed by the same stem cell population. The need to maintain balanced diversity of cell types in an unceasingly regenerating tissue demands intricate mechanisms of spatial or temporal cell fate control. Recent advances in single-cell sequencing, spatio-temporal profiling and organoid technology have shed new light on the intricate micro-structure of the intestinal epithelium and on the mechanisms that maintain it. This led to the discovery of unexpected plasticity, zonation along the crypt-villus axis and new mechanism of self-organization. However, not only the epithelium, but also the underlying mesenchyme is distinctly structured. Several new studies have explored the intestinal stroma with single cell resolution and unveiled important interactions with the epithelium that are crucial for intestinal function and regeneration. In this review, we will discuss these recent findings and highlight the technologies that lead to their discovery. We will examine strengths and limitations of each approach and consider the wider impact of these results on our understanding of the intestine in health and disease.
Keywords: cancer; differentiation; intestine; organoid; plasticity; regeneration; single cell; stem cell.
Copyright © 2021 Bonis, Rossell and Gehart.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

