Understanding Immune Responses to Surgical Transplant Procedures in Stevens Johnsons Syndrome Patients
- PMID: 34095169
- PMCID: PMC8175970
- DOI: 10.3389/fmed.2021.656998
Understanding Immune Responses to Surgical Transplant Procedures in Stevens Johnsons Syndrome Patients
Abstract
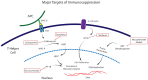
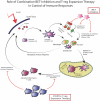
Stevens Johnsons syndrome (SJS) is a mucocutaneous disorder caused by an autoimmune response most commonly to medications. Unless it is properly managed in the acute setting, this entity can affect the ocular surface causing chronic cicatrizing conjunctivitis with limbal stem cell deficiency and lid anomalies which ultimately result in corneal opacities that may limit patients' visual acuity. When this stage is reached, some patients might need to undergo some form of corneal and/or limbal stem cell transplantation that exposes an already sensitized immune system to a new alloantigen. While the innate immunity plays a role in corneal graft survival, adaptive immune responses play a major part in corneal graft rejection and failure, namely through CD4+ T cell lymphocytes. Hence, the management of the immune response to surgical transplant procedures in SJS patients, involves a dual approach that modulates the inflammatory response to a new alloantigen in the context of an autoimmune sensitized patient. This review will explore and discuss current perspectives and future directions in the field of ocular immunology on how to manage SJS immune responses to ocular surgical procedures, reviewing systemic and local immunosuppressive therapies and protocols to adequately manage this debilitating condition.
Keywords: Stevens Johnsons; corneal transplant; high risk corneal transplantation; immunosuppression; limbal stem cell transplant.
Copyright © 2021 Soifer, Mousa, Levy and Perez.
Conflict of interest statement
RBL is a compensated consultant/advisory board member for and equity holder in Heat Biologics. VLP has worked as a compensated consultant for Alcon, Eyegate, Oculis, Novartis, Trefoil, Quidel, Dompe and is a board member of OBTears. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Limbal stem cell transplantation: an evidence-based analysis.Ont Health Technol Assess Ser. 2008;8(7):1-58. Epub 2008 Oct 1. Ont Health Technol Assess Ser. 2008. PMID: 23074512 Free PMC article.
-
Stevens-Johnson syndrome: The role of an ophthalmologist.Surv Ophthalmol. 2016 Jul-Aug;61(4):369-99. doi: 10.1016/j.survophthal.2016.01.004. Epub 2016 Jan 30. Surv Ophthalmol. 2016. PMID: 26829569 Review.
-
Corneal Limbal Stem Cell Deficiency in Children with Stevens-Johnson Syndrome.Am J Ophthalmol. 2019 Mar;199:1-8. doi: 10.1016/j.ajo.2018.10.016. Epub 2018 Oct 19. Am J Ophthalmol. 2019. PMID: 30347185
-
Epithelial transplantation for the management of severe ocular surface disease.Trans Am Ophthalmol Soc. 1996;94:677-743. Trans Am Ophthalmol Soc. 1996. PMID: 8981714 Free PMC article.
-
[Clinical management of severe ocular surface disease].Klin Monbl Augenheilkd. 2005 Jul;222(7):533-51. doi: 10.1055/s-2005-858427. Klin Monbl Augenheilkd. 2005. PMID: 16034721 Review. German.
Cited by
-
Corneal Epithelial Stem Cells-Physiology, Pathophysiology and Therapeutic Options.Cells. 2021 Sep 3;10(9):2302. doi: 10.3390/cells10092302. Cells. 2021. PMID: 34571952 Free PMC article. Review.
-
Ocular Surface Inflammatory Disorders (OSID): A Collective of Systemic Etiologies Which Cause or Amplify Dry Eye Syndrome.Front Med (Lausanne). 2022 Jul 6;9:949202. doi: 10.3389/fmed.2022.949202. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35872765 Free PMC article.
References
-
- Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bavinck JNB, et al. . Stevens–Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-Study. J Invest Dermatol. (2008) 128:35–44. 10.1038/sj.jid.5701033 - DOI - PubMed
-
- Chan HL, Stern RS, Arndt KA, Langlois J, Jick SS, Jick H, et al. . The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. (1990) 126:43–7. 10.1001/archderm.126.1.43 - DOI - PubMed
-
- Rzany B, Mockenhaupt M, Baur S, Schröder W, Stocker U, Mueller J, et al. . Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol. (1996) 49:769–73. 10.1016/0895-4356(96)00035-2 - DOI - PubMed
-
- Diphoorn J, Cazzaniga S, Gamba C, Schroeder J, Citterio A, Rivolta AL, et al. . Incidence, causative factors and mortality rates of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in northern Italy: data from the REACT registry: incidence of SJS and TEN in northern Italy. Pharmacoepidemiol Drug Saf. (2016) 25:196–203. 10.1002/pds.3937 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

