A Novel GH Family 20 β-N-acetylhexosaminidase With Both Chitosanase and Chitinase Activity From Aspergillus oryzae
- PMID: 34095233
- PMCID: PMC8170477
- DOI: 10.3389/fmolb.2021.684086
A Novel GH Family 20 β-N-acetylhexosaminidase With Both Chitosanase and Chitinase Activity From Aspergillus oryzae
Abstract
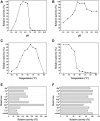
Aminooligosaccharides possess various biological activities and can exploit wide applications in food, pharmaceutical and cosmetic industries. Commercial aminooligosaccharides are often prepared by the hydrolysis of chitin and chitosan. In this study, a novel GH family 20 β-N-acetylhexosaminidases gene named AoNagase was cloned from Aspergillus oryzae and expressed in Pichia pastoris. The purified AoNagase had maximal activity at pH 5.5 and 65°C. It exhibited good pH stability in the range of pH 6.0-7.5 and at temperatures below 50°C. AoNagase was capable of hydrolyzing not only colloidal chitosan (508.26 U/mg) but also chitin (29.78 U/mg). The kinetic parameters (K m and V max ) of AoNagase were 1.51 mM, 1106.02 U/mg for chitosan and 0.41 mM, 40.31 U/mg for colloidal chitin. To our knowledge, AoNagase is the first GH family 20 β-N-acetylhexosaminidase capable of hydrolyzing both chitosan and chitin. AoNagase is an endo-type β-N-acetylhexosaminidases and can potentially be used for the manufacturing of aminooligosaccharides.
Keywords: aminooligosaccharide; chitin; chitosan; glycoside hydrolase family 20; β-N-acetylhexosaminidase.
Copyright © 2021 Qu, Zhang, Qin, Fan, Jiang and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

