Quantifying the Impact of Nasopharyngeal Specimen Quality on Severe Acute Respiratory Syndrome Coronavirus 2 Test Performance
- PMID: 34095340
- PMCID: PMC8136075
- DOI: 10.1093/ofid/ofab235
Quantifying the Impact of Nasopharyngeal Specimen Quality on Severe Acute Respiratory Syndrome Coronavirus 2 Test Performance
Abstract
Background: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse-transcription polymerase chain reaction (RT-PCR) cycle threshold (Ct) has been used to estimate quantitative viral load, with the goal of targeting isolation precautions for individuals with coronavirus disease 2019 (COVID-19) and guiding public health interventions. However, variability in specimen quality can alter the Ct values obtained from SARS-CoV-2 clinical assays. We sought to define how variable nasopharyngeal (NP) swab quality impacts clinical SARS-CoV-2 test sensitivity.
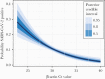
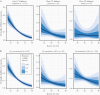
Methods: We performed amplification of a human gene target (β-actin) in parallel with a clinical RT-PCR targeting the SARS-CoV-2 ORF1ab gene for 1282 NP specimens collected from patients with clinical concern for COVID-19. We evaluated the relationship between NP specimen quality, characterized by late Ct values for the human gene target β-actin Ct, and the probability of SARS-CoV-2 detection via logistic regression, as well as the linear relationship between SARS-CoV-2 and β-actin Ct.
Results: Low-quality NP swabs are less likely to detect SARS-CoV-2 (odds ratio, 0.607 [95% credible interval {CrI}, .487-.753]). We observed a positive linear relationship between SARS-CoV-2 and β-actin Ct values (slope, 0.181 [95% CrI, .097-.264]), consistent with a reduction in detection of 0.181 cycles for each additional cycle of the β-actin target. COVID-19 disease severity was not associated with β-actin Ct values.
Conclusions: Variability in NP specimen quality significantly impacts the performance of clinical SARS-CoV-2 assays, and caution should be taken when interpreting quantitative SARS-CoV-2 Ct results. If unrecognized, low-quality NP specimens, which are characterized by a low level of amplifiable human DNA target, may limit the successful application of SARS-CoV-2 Ct values to direct infection control and public health interventions.
Keywords: SARS-CoV-2; cycle threshold; test sensitivity.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
Update of
-
Impact of Nasopharyngeal Specimen Quality on SARS-CoV-2 Test Sensitivity.medRxiv [Preprint]. 2020 Dec 11:2020.12.09.20246520. doi: 10.1101/2020.12.09.20246520. medRxiv. 2020. Update in: Open Forum Infect Dis. 2021 May 12;8(6):ofab235. doi: 10.1093/ofid/ofab235. PMID: 33330893 Free PMC article. Updated. Preprint.
References
-
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

