Mechanistic model for production of recombinant adeno-associated virus via triple transfection of HEK293 cells
- PMID: 34095346
- PMCID: PMC8143981
- DOI: 10.1016/j.omtm.2021.04.006
Mechanistic model for production of recombinant adeno-associated virus via triple transfection of HEK293 cells
Abstract
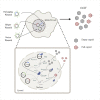
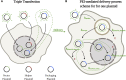
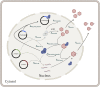
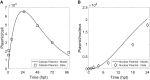
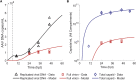
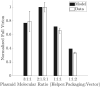
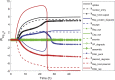
Manufacturing of recombinant adeno-associated virus (rAAV) viral vectors remains challenging, with low yields and low full:empty capsid ratios in the harvest. To elucidate the dynamics of recombinant viral production, we develop a mechanistic model for the synthesis of rAAV viral vectors by triple plasmid transfection based on the underlying biological processes derived from wild-type AAV. The model covers major steps starting from exogenous DNA delivery to the reaction cascade that forms viral proteins and DNA, which subsequently result in filled capsids, and the complex functions of the Rep protein as a regulator of the packaging plasmid gene expression and a catalyst for viral DNA packaging. We estimate kinetic parameters using dynamic data from literature and in-house triple transient transfection experiments. Model predictions of productivity changes as a result of the varied input plasmid ratio are benchmarked against transfection data from the literature. Sensitivity analysis suggests that (1) the poorly coordinated timeline of capsid synthesis and viral DNA replication results in a low ratio of full virions in harvest, and (2) repressive function of the Rep protein could be impeding capsid production at a later phase. The analyses from the mathematical model provide testable hypotheses for evaluation and reveal potential process bottlenecks that can be investigated.
Keywords: AAV-based gene therapy; adeno-associated virus; biomanufacturing; gene therapy; gene-therapy manufacturing; mechanistic modeling; mechanistic understanding; transfection; viral vector; viral vector manufacturing.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Multidose transient transfection of human embryonic kidney 293 cells modulates recombinant adeno-associated virus2/5 Rep protein expression and influences the enrichment fraction of filled capsids.Biotechnol Bioeng. 2024 Dec;121(12):3694-3714. doi: 10.1002/bit.28828. Epub 2024 Aug 23. Biotechnol Bioeng. 2024. PMID: 39176568
-
Perfusion-Based Production of rAAV via an Intensified Transient Transfection Process.Biotechnol Bioeng. 2025 Jun;122(6):1424-1440. doi: 10.1002/bit.28967. Epub 2025 Mar 18. Biotechnol Bioeng. 2025. PMID: 40103325 Free PMC article.
-
Recombinant adeno-associated virus type 2 replication and packaging is entirely supported by a herpes simplex virus type 1 amplicon expressing Rep and Cap.J Virol. 1997 Nov;71(11):8780-9. doi: 10.1128/JVI.71.11.8780-8789.1997. J Virol. 1997. PMID: 9343238 Free PMC article.
-
Cellular pathways of recombinant adeno-associated virus production for gene therapy.Biotechnol Adv. 2021 Jul-Aug;49:107764. doi: 10.1016/j.biotechadv.2021.107764. Epub 2021 May 3. Biotechnol Adv. 2021. PMID: 33957276 Review.
-
Development of Stable Packaging and Producer Cell Lines for the Production of AAV Vectors.Microorganisms. 2024 Feb 13;12(2):384. doi: 10.3390/microorganisms12020384. Microorganisms. 2024. PMID: 38399788 Free PMC article. Review.
Cited by
-
Temporal insights into molecular and cellular responses during rAAV production in HEK293T cells.Mol Ther Methods Clin Dev. 2024 Jun 8;32(3):101278. doi: 10.1016/j.omtm.2024.101278. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39022743 Free PMC article.
-
Recent Advances in Therapeutics and Manufacturing Processes of Recombinant Adeno-Associated Virus for the Treatment of Lung Diseases.Curr Gene Ther. 2025;25(3):237-256. doi: 10.2174/0115665232294935240826061311. Curr Gene Ther. 2025. PMID: 39225214 Review.
-
Iterative hybrid model based optimization of rAAV production.Biotechnol Prog. 2025 Jul-Aug;41(4):e70006. doi: 10.1002/btpr.70006. Epub 2025 Mar 24. Biotechnol Prog. 2025. PMID: 40129076 Free PMC article.
-
Machine Learning and Hybrid Methods for Metabolic Pathway Modeling.Methods Mol Biol. 2023;2553:417-439. doi: 10.1007/978-1-0716-2617-7_18. Methods Mol Biol. 2023. PMID: 36227553 Review.
-
Liter-scale manufacturing of shelf-stable plasmid DNA/PEI transfection particles for viral vector production.Mol Ther Methods Clin Dev. 2024 Jan 22;32(1):101194. doi: 10.1016/j.omtm.2024.101194. eCollection 2024 Mar 14. Mol Ther Methods Clin Dev. 2024. PMID: 38352269 Free PMC article.
References
-
- Alliance for Regenerative Medicine . 2020. Advancing Innovation During COVID-19: ARM Global Regenerative Medicine & Advanced Therapy Sector Report, H1.https://alliancerm.org/sector-report/h1-2020-report/
-
- U.S. Food and Drug Administration . 2019. Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies. January 15, 2019.https://www.fda.gov/news-events/press-announcements/statement-fda-commis...
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

