Spatio-spectral relationships between pathological neural dynamics and cognitive impairment along the Alzheimer's disease spectrum
- PMID: 34095434
- PMCID: PMC8165730
- DOI: 10.1002/dad2.12200
Spatio-spectral relationships between pathological neural dynamics and cognitive impairment along the Alzheimer's disease spectrum
Erratum in
-
Erratum.Alzheimers Dement (Amst). 2022 Feb 11;14(1):e12257. doi: 10.1002/dad2.12257. eCollection 2022. Alzheimers Dement (Amst). 2022. PMID: 35169609 Free PMC article.
Abstract
Introduction: Numerous studies have described aberrant patterns of rhythmic neural activity in patients along the Alzheimer's disease (AD) spectrum, yet the relationships between these pathological features and cognitive decline are uncertain.
Methods: We acquired magnetoencephalography (MEG) data from 38 amyloid-PET biomarker-confirmed patients on the AD spectrum and a comparison group of biomarker-negative cognitively normal (CN) healthy adults, alongside an extensive neuropsychological battery.
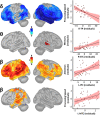
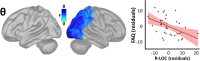
Results: By modeling whole-brain rhythmic neural activity with an extensive neuropsychological profile in patients on the AD spectrum, we show that the spectral and spatial features of deviations from healthy adults in neural population-level activity inform their relevance to domain-specific neurocognitive declines.
Discussion: Regional oscillatory activity represents a sensitive metric of neuronal pathology in patients on the AD spectrum. By considering not only the spatial, but also the spectral, definitions of cortical neuronal activity, we show that domain-specific cognitive declines can be better modeled in these individuals.
Keywords: neural oscillations; neuropsychology; resting‐state magnetoencephalography.
© 2021 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no competing conflicts of interest, financial or otherwise.
Figures


References
-
- Collie A, Maruff P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neurosci Biobehav Rev. 2000;24(3):365‐374. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
