Alzheimer's disease drug development pipeline: 2021
- PMID: 34095440
- PMCID: PMC8145448
- DOI: 10.1002/trc2.12179
Alzheimer's disease drug development pipeline: 2021
Abstract
Introduction: The number of individuals worldwide with Alzheimer's disease (AD) is growing at a rapid rate. New treatments are urgently needed. We review the current pipeline of drugs in clinical trials for the treatment of AD.
Methods: We interrogated ClinicalTrials.gov, the federal registry of clinical trials to identify drugs in trials.
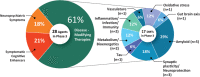
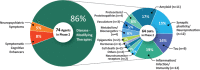
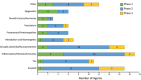
Results: There are 126 agents in 152 trials assessing new therapies for AD: 28 treatments in Phase 3 trials, 74 in Phase 2, and 24 in Phase 1. The majority of drugs in trials (82.5%) target the underlying biology of AD with the intent of disease modification; 10.3% are putative cognitive enhancing agents; and 7.1% are drugs being developed to reduce neuropsychiatric symptoms.
Discussion: This pipeline analysis shows that target biological processes are more diversified, biomarkers are more regularly used, and repurposed agents are being explored to determine their utility for the treatment of AD.
Keywords: Alzheimer's disease; Common Alzheimer's Disease Research Ontology (CADRO); National Institutes of Health; amyloid; biomarkers; clinical trials; drug development; inflammation; pharmaceutical companies; repurposed drugs; tau.
© 2021 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals, Inc. on behalf of Alzheimer's Association.
Conflict of interest statement
JC has provided consultation to Acadia, Alkahest, AriBio, Avanir, Axsome, Behren Therapeutics, Biogen, Cassava, Cerecin, Cerevel, Cortexyme, EIP Pharma, Eisai, Foresight, GemVax, Genentech, Green Valley, Grifols, Janssen, Jazz, Merck, Novo Nordisk, Otsuka, ReMYND, Resverlogix, Roche, Signant Health, Sunovion, Suven, United Neuroscience, and Unlearn AI pharmaceutical and assessment companies. Dr. Cummings has stock options in ADAMAS, AnnovisBio, MedAvante, BiOasis, and United Neuroscience. Dr. Cummings owns the copyright of the Neuropsychiatric Inventory. Dr Cummings is supported by NIGMS grant P20GM109025; NINDS grant U01NS093334; NIA grant R01AG053798; and NIA grant P20AG068053. GL is a full‐time employee of Biogen. KZ provides consultation to Green Valley Pharmaceuticals. JF has no disclosures. KT has no disclosures.
Figures

References
-
- Alzheimer's Association . 2020 Alzheimer's disease facts and figures. Alzheimer Dement. 2020;16:391‐460.
-
- Food and Drug Administration . Early Alzheimer's disease: developing drugs for treatment guidance for industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER; ). 2018.
-
- International Alzheimer's and Related Dementia Research Portfolio; Accessed January 4, 2021; https://iadrp.nia.nih.gov/about/cadro
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
