Conversion from cilostazol to OPC-13015 linked to mitigation of cognitive impairment
- PMID: 34095441
- PMCID: PMC8158162
- DOI: 10.1002/trc2.12182
Conversion from cilostazol to OPC-13015 linked to mitigation of cognitive impairment
Abstract
Introduction: Cilostazol may be a novel therapeutic agent for Alzheimer's disease. Its metabolite, OPC-13015, has a stronger inhibitory effect on type 3 phosphodiesterase than cilostazol.
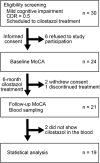
Methods: We prospectively enrolled patients with mild cognitive impairment to whom cilostazol was newly prescribed. Patients underwent the Montreal Cognitive Assessment (MoCA) twice, at a 6-month interval. Plasma cilostazol, OPC-13015, OPC-13213, and OPC-13217 concentrations were determined using liquid chromatography-tandem mass spectrometry.
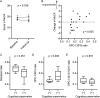
Results: MoCA score changes from baseline to the 6-month visit were positively correlated with ratios of OPC-13015 to cilostazol and total metabolites (n = 19, P = .005). Patients with higher ratios of OPC-13015 (≥0.18, median value; n = 10) had significantly higher MoCA scores (P = .036) than patients with lower ratios (the ratio <0.18, n = 9). The absolute value of OPC-13015 concentration in blood was also higher in patients with preserved cognitive function (P = .033).
Discussion: Blood OPC-13015 levels may be a predictive biomarker of cilostazol treatment for Alzheimer's disease.
Keywords: Alzheimer's disease; OPC‐13015; cilostazol; drug repositioning; mild cognitive impairment; stratified medicine.
© 2021 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals, Inc. on behalf of Alzheimer's Association.
Conflict of interest statement
Mr. Kawakami and Mr. Yamamoto are employees of Shimadzu Corporation, an analytical instrument manufacturer that is not involved in the sale of cilostazol. Dr. Ihara has a patent concerning cilostazol (WO2013187075A1) titled “Prophylactic and/or therapeutic agent for mild cognitive impairment.” Dr. Saito, Mr. Kawakami, Mr. Yamamoto, and Dr. Ihara have applied for a patent concerning the usefulness of evaluating the levels of cilostazol and its metabolites. In addition, Dr. Ihara reports grants from Panasonic, BMS, and Otsuka Pharmaceutical, and personal fees from Daiichi Sankyo Co Ltd, Eisai, and Bayer, which were not involved in the current work. The other authors declare no competing financial interests.
Figures


References
LinkOut - more resources
Full Text Sources
