Efficacy of Hybrid Closed-Loop Insulin Delivery System in a Hospital Setting: A Case Series
- PMID: 34095484
- PMCID: PMC8165117
- DOI: 10.1016/j.aace.2020.12.013
Efficacy of Hybrid Closed-Loop Insulin Delivery System in a Hospital Setting: A Case Series
Abstract
Objective: We report a case series of 4 patients with type 1 diabetes who used hybrid closed-loop insulin pumps (Medtronic MiniMed 670 G) during hospitalization.
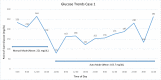
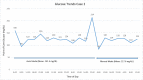
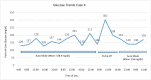
Methods: Clinical data and point-of-care glucose values are presented for each patient. Glucose values are shown graphically while in manual mode as well as in auto mode.
Results: The first case was a 30-year-old man admitted for pancreatitis. Mean point-of-care blood glucose was 165.7 mg/dL while in auto mode, without hypoglycemia, compared with 221 mg/dL while in manual mode. The second case was a 28-year-old woman who was admitted for a laparoscopic cholecystectomy. Mean point-of-care blood glucose in auto mode was 131.3 mg/dL, without hypoglycemia, compared with 117.6 mg/dL while in manual mode. The third case was a 46-year-old man admitted to the intensive care unit for influenzal pneumonia. Mean point-of-care blood glucose in auto mode was 159.1 mg/dL without hypoglycemia, compared with 218.5 mg/dL while in manual mode. The fourth case was a 60-year-old man who remained in auto mode throughout his hospitalization except for a period when he removed his pump for an endoscopic retrograde cholangiopancreatography and endoscopic ultrasound. His mean point-of-care blood glucose while in auto mode was 156.8 mg/dL without hypoglycemia.
Conclusion: These case reports support the use of hybrid closed-loop insulin-pump therapy in the inpatient setting to maintain inpatient glycemic targets and avoid hypoglycemia when part of an institution-sanctioned strategy for safe use of insulin pumps that includes point-of-care blood glucose monitoring.
Keywords: CGM, continuous glucose monitor; FDA, Food and Drug Administration; HCL, hybrid closed-loop; MARD, mean absolute relative difference; T1DM, type 1 diabetes mellitus.
© 2021 AACE. Published by Elsevier Inc.
Figures
References
-
- Yeh H.C., Brown T.T., Maruthur N. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336–347. - PubMed
-
- US Food and Drug Administration FDA approves first automated insulin delivery device for type 1 diabetes. https://www.fda.gov/news-events/press-announcements/fda-approves-first-a...
-
- Ly T.T., Roy A., Grosman B. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in Type 1 diabetes at diabetes camp. Diabetes Care. 2015;38(7):1205–1211. - PubMed
Publication types
LinkOut - more resources
Full Text Sources