Platinum-crosslinking polymeric nanoparticle for synergetic chemoradiotherapy of nasopharyngeal carcinoma
- PMID: 34095627
- PMCID: PMC8164009
- DOI: 10.1016/j.bioactmat.2021.05.010
Platinum-crosslinking polymeric nanoparticle for synergetic chemoradiotherapy of nasopharyngeal carcinoma
Abstract
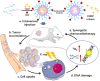
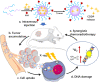
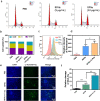
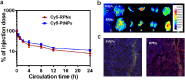
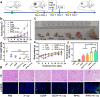
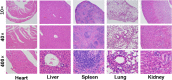
Despite extensive use of radiotherapy in nasopharyngeal carcinoma (NPC) treatment because of its high radiosensitivity, there have been huge challenges in further improving therapeutic effect, meanwhile obviously reducing radiation damage. To this end, synergistic chemoradiotherapy has emerged as a potential strategy for highly effective NPC therapy. Here, we developed RGD-targeted platinum-based nanoparticles (RGD-PtNPs, denoted as RPNs) to achieve targeted chemoradiotherapy for NPC. Such nanoparticles consist of an RGD-conjugated shell and a cis-platinum (CDDP) crosslinking core. Taking advantage of RGD, the RPNs may effectively accumulate in tumor, penetrate into tumor tissues and be taken by cancer cells, giving rise to a high delivery efficiency of CDDP. When they are fully enriched in tumor sites, the CDDP loaded RPNs can act as radiotherapy sensitizer and chemotherapy agents. By means of X-ray-promoted tumor cell uptake of nanoparticle and CDDP-induced cell cycle arrest in radiation-sensitive G2/M phases, RPNs may offer remarkable therapeutic outcome in the synergistic chemoradiotherapy for NPC.
Keywords: Chemoradiotherapy; Nasopharyngeal carcinoma (NPC); Polymeric nanoparticles; Precise treatment.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Chua M.L.K., Wee J.T.S., Hui E.P., Chan A.T.C. Lancet. 2016;387:1012–1024. - PubMed
-
- Wei W.I., Sham J.S.T. Lancet. 2005;365:2041–2054. - PubMed
-
- Chen Y.-P., Chan A.T.C., Le Q.-T., Blanchard P., Sun Y., Ma J. Lancet. 2019;394:64–80. - PubMed
-
- Blanchard P., Lee A., Marguet S., Leclercq J., Ng W.T., Ma J., Chan A.T.C., Huang P.-Y., Benhamou E., Zhu G., Chua D.T.T., Chen Y., Mai H.-Q., Kwong D.L.W., Cheah S.L., Moon J., Tung Y., Chi K.-H., Fountzilas G., Zhang L., Hui E.P., Lu T.-X., Bourhis J., Pignon J.P. Lancet Oncol. 2015;16:645–655. - PubMed
LinkOut - more resources
Full Text Sources

