Downstream Paclitaxel Released Following Drug-Coated Balloon Inflation and Distal Limb Wound Healing in Swine
- PMID: 34095632
- PMCID: PMC8165120
- DOI: 10.1016/j.jacbts.2021.01.012
Downstream Paclitaxel Released Following Drug-Coated Balloon Inflation and Distal Limb Wound Healing in Swine
Abstract
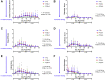
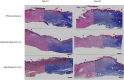
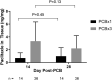
The authors evaluated the presence of paclitaxel and healing of distal hind limb wounds created in 27 swine using biopsy punches followed by paclitaxel-coated balloon (PCB) use in the iliofemoral arteries of healthy swine. After 14 and 28 days, no differences were seen in time course, appearance, and histopathology of wound healing between the single or triple PCB and uncoated balloon treatment despite clinically relevant paclitaxel concentrations in the skin adjacent to the healing wounds. Presence of paclitaxel downstream from the PCB treatment site does not impair the wound healing response of preexisting distal cutaneous lesions in healthy swine.
Keywords: BTK, below the knee; CLI, critical limb ischemia; PCB, paclitaxel coated balloon; PTA, percutaneous transluminal angioplasty; SFA, superficial femoral artery; histopathology; paclitaxel-coated balloon; swine; translational; wound healing.
© 2021 The Authors.
Conflict of interest statement
Ms. Crookall, and Drs. Schulz-Jander, Tunev, and Melder are full-time employees of Medtronic PLC, the manufacturer of the IN.PACT Admiral product evaluated in the study. Dr. Granada is the CEO, and Dr. Kaluza is an employee of the Cardiovascular Research Foundation which receives research and educational grant support from Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





Comment in
-
Can Paclitaxel Coated Balloons Have a Deep Impact on Critical Limb Ischemia?JACC Basic Transl Sci. 2021 May 24;6(5):428-430. doi: 10.1016/j.jacbts.2021.04.003. eCollection 2021 May. JACC Basic Transl Sci. 2021. PMID: 34101768 Free PMC article. No abstract available.
References
-
- Rosenfield K., Jaff M.R., White C.J. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. - PubMed
-
- Brodmann M., Werner M., Meyer D.R. Sustainable antirestenosis effect with a low-dose drug-coated balloon: the ILLUMENATE European randomized clinical trial 2-year results. J Am Coll Cardiol Intv. 2018;11:2357–2364. - PubMed
-
- Cremers B., Speck U., Kaufels N. Drug-eluting balloon: very short-term exposure and overlapping. Thromb Haemost. 2009;101:201–206. - PubMed
-
- Gongora C.A., Shibuya M., Wessler J.D. Impact of paclitaxel dose on tissue pharmacokinetics and vascular healing: a comparative drug-coated balloon study in the familial hypercholesterolemic swine model of superficial femoral in-stent restenosis. J Am Coll Cardiol Intv. 2015;8:1115–1123. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

