Selective Interleukin-6 Trans-Signaling Blockade Is More Effective Than Panantagonism in Reperfused Myocardial Infarction
- PMID: 34095633
- PMCID: PMC8165121
- DOI: 10.1016/j.jacbts.2021.01.013
Selective Interleukin-6 Trans-Signaling Blockade Is More Effective Than Panantagonism in Reperfused Myocardial Infarction
Abstract
Interleukin (IL)-6 is an emerging therapeutic target in myocardial infarction (MI). IL-6 has 2 distinct signaling pathways: trans-signaling, which mediates inflammation, and classic signaling, which also has anti-inflammatory effects. The novel recombinant fusion protein sgp130Fc achieves exclusive trans-signaling blockade, whereas anti-IL-6 antibodies (Abs) result in panantagonism. In a rat model of reperfused MI, sgp130Fc, but not anti-IL-6-Ab, attenuated neutrophil and macrophage infiltration into the myocardium, reduced infarct size, and preserved cardiac function 28 days after MI. These data demonstrate the efficacy of exclusive IL-6 trans-signaling blockade and support further investigation of sgp130Fc as a potential novel therapy in MI.
Keywords: AAR, area at risk; Ab, antibody; CCL, C-C motif chemokine ligand; CMR, cardiac magnetic resonance; CXCL, C-X-C motif ligand; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; IS, infarct size; LGE, late-gadolinium enhancement; LVEF, left ventricular ejection fraction; MHC, major histocompatibility complex; MI, myocardial infarction; NSTEMI, non–ST-segment-elevation MI; RCAEC, rat coronary artery endothelial cell; STEMI, ST-segment-elevation MI; TCZ, tocilizumab; Trop-T, troponin T; c-caspase-3, cleaved caspase-3; inflammation; interleukin-6; myocardial infarction; reperfusion; sIL-6R, soluble IL-6 receptor.
© 2021 The Authors.
Conflict of interest statement
This work was supported by the Wellcome Trust (108735/Z/15/Z to Dr. George and 212937/Z/18/Z to Dr. Stuckey), the British Heart Foundation (FS/15/33/31608 and RM/17/1/33377 to Dr. Stuckey), the Medical Research Council (MR/R026416/1 to Dr. Stuckey), the National Institute for Health Research (Senior Investigator: Dr, Hingorani), and a King Scholarship of Malaysia (Ms. Jasmin). Dr. Woollard is an employee of AstraZeneca. No funding or support was received from AstraZeneca. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
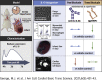
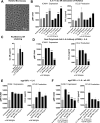
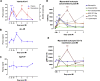
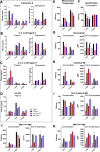
Comment in
-
Targeting IL-6 Trans-Signaling: Amplifying the Benefits of IL-6 Inhibition in Myocardial Infarction.JACC Basic Transl Sci. 2021 May 24;6(5):444-446. doi: 10.1016/j.jacbts.2021.01.007. eCollection 2021 May. JACC Basic Transl Sci. 2021. PMID: 34102669 Free PMC article.
References
-
- Romano M., Sironi M., Toniatti C. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

