Integrating Somatic and Germline Next-Generation Sequencing Into Routine Clinical Oncology Practice
- PMID: 34095711
- PMCID: PMC8169076
- DOI: 10.1200/PO.20.00513
Integrating Somatic and Germline Next-Generation Sequencing Into Routine Clinical Oncology Practice
Abstract
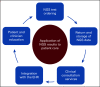
Next-generation sequencing (NGS) is rapidly expanding into routine oncology practice. Genetic variations in both the cancer and inherited genomes are informative for hereditary cancer risk, prognosis, and treatment strategies. Herein, we focus on the clinical perspective of integrating NGS results into patient care to assist with therapeutic decision making. Five key considerations are addressed for operationalization of NGS testing and application of results to patient care as follows: (1) NGS test ordering and workflow design; (2) result reporting, curation, and storage; (3) clinical consultation services that provide test interpretations and identify opportunities for molecularly guided therapy; (4) presentation of genetic information within the electronic health record; and (5) education of providers and patients. Several of these key considerations center on informatics tools that support NGS test ordering and referencing back to the results for therapeutic purposes. Clinical decision support tools embedded within the electronic health record can assist with NGS test utilization and identifying opportunities for targeted therapy including clinical trial eligibility. Challenges for project and change management in operationalizing NGS-supported, evidence-based patient care in the context of current information technology systems with appropriate clinical data standards are discussed, and solutions for overcoming barriers are provided.
© 2021 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). J. Kevin Hicks Consulting or Advisory Role: Quest Diagnostics, 23andMe Research Funding: OneOmeKaren K. Fields Stock and Other Ownership Interests: Pfizer, AbbVie Honoraria: NCCN, NACCME, Pacific Group on Business, HMP, MJH Healthcare Holdings LLC Consulting or Advisory Role: United Health Group Speakers' Bureau: Genentech/Roche Travel, Accommodations, Expenses: Genentech/Roche, CBI, Discern Health, Tapestry, NACCME, Cigna Health Care, FLASCOJhanelle E. Gray Consulting or Advisory Role: Bristol Myers Squibb, EMD Serono, Inivata, Merck Sharp & Dohme, Axiom Healthcare Strategies, Novartis, AstraZeneca, Blueprint Medicines, Lilly, Sanofi, Janssen Scientific Affairs Research Funding: Array BioPharma, Merck, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Genentech/Roche, G1 Therapeutics, Novartis, Pfizer, Ludwig Institute for Cancer Research Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck Sharp & Dohme, Inivata, Merck, EMD Serono, NovartisBryan McIver Honoraria: Eisai, Loxo/Lilly, Blueprint Medicines, Exelixis Consulting or Advisory Role: Eisai, Loxo/Lilly, Exelixis Speakers' Bureau: Loxo/LillyMandy F. O'Leary Stock and Other Ownership Interests: Bristol Myers SquibbRanda M. Perkins Stock and Other Ownership Interests: Progyny Other Relationship: Centene Open Payments Link: https://openpaymentsdata.cms.gov/physician/263299Jamie K. Teer Patents, Royalties, Other Intellectual Property: Patent application: Large Data Set Negative Information Storage ModelJoseph Markowitz Stock and Other Ownership Interests: Intel, Aflac, CVS, Amdocs, Consolidated Edison Honoraria: Springer Consulting or Advisory Role: Idera, Newlink Genetics, Array Biopharma Research Funding: Reata Pharmaceuticals, Macrogenics, Morphogenesis, Genoptix, Idera, Jackson Laboratory for Genomic Medicine, Merck Other Relationship: SpringerDana E. Rollison Leadership: NanoString Technologies Stock and Other Ownership Interests: NanoString Technologies Patents, Royalties, Other Intellectual Property: I am a co-inventor on a provisional patent application filed in July of 2020. The patent pertains to the use of spectrophotometer-measured UV radiation exposure in combination with regulatory T-cells measured in circulation to predict risk of subsequent skin cancer Travel, Accommodations, Expenses: Caserta Analytics Inc (not a healthcare company specifically, but they do business with healthcare companies), NanoString Technologies No other potential conflicts of interest were reported.
Figures
References
-
- US Food and Drug Administration : Table of Pharmacogenomic Biomarkers in Drug Labeling. Silver Spring, MD, US Food and Drug Administration; 2020
-
- US Food and Drug Administration : Table of Pharmacogenetic Associations. Silver Spring, MD, US Food and Drug Administration; 2020
-
- Ettinger DS, Aisner DL, Wood DE, et al. : NCCN guidelines insights: Non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw 16:807-8212018 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

