Serial Monitoring of Circulating Tumor DNA by Next-Generation Gene Sequencing as a Biomarker of Response and Survival in Patients With Advanced NSCLC Receiving Pembrolizumab-Based Therapy
- PMID: 34095713
- PMCID: PMC8169078
- DOI: 10.1200/PO.20.00321
Serial Monitoring of Circulating Tumor DNA by Next-Generation Gene Sequencing as a Biomarker of Response and Survival in Patients With Advanced NSCLC Receiving Pembrolizumab-Based Therapy
Abstract
Although the majority of patients with metastatic non-small-cell lung cancer (mNSCLC) lacking a detectable targetable mutation will receive pembrolizumab-based therapy in the frontline setting, predicting which patients will experience a durable clinical benefit (DCB) remains challenging.
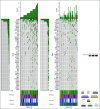
Materials and methods: Patients with mNSCLC receiving pembrolizumab monotherapy or in combination with chemotherapy underwent a 74-gene next-generation sequencing panel on blood samples obtained at baseline and at 9 weeks. The change in circulating tumor DNA levels on-therapy (molecular response) was quantified using a ratio calculation with response defined by a > 50% decrease in mean variant allele fraction. Patient response was assessed using RECIST 1.1; DCB was defined as complete or partial response or stable disease that lasted > 6 months. Progression-free survival and overall survival were recorded.
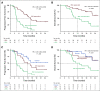
Results: Among 67 patients, 51 (76.1%) had > 1 variant detected at a variant allele fraction > 0.3% and thus were eligible for calculation of molecular response from paired baseline and 9-week samples. Molecular response values were significantly lower in patients with an objective radiologic response (log mean 1.25% v 27.7%, P < .001). Patients achieving a DCB had significantly lower molecular response values compared to patients with no durable benefit (log mean 3.5% v 49.4%, P < .001). Molecular responders had significantly longer progression-free survival (hazard ratio, 0.25; 95% CI, 0.13 to 0.50) and overall survival (hazard ratio, 0.27; 95% CI, 0.12 to 0.64) compared with molecular nonresponders.
Conclusion: Molecular response assessment using circulating tumor DNA may serve as a noninvasive, on-therapy predictor of response to pembrolizumab-based therapy in addition to standard of care imaging in mNSCLC. This strategy requires validation in independent prospective studies.
© 2021 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Jeffrey C. ThompsonConsulting or Advisory Role: OncoCyte, AstraZeneca, Guardant HealthErica L. CarpenterHonoraria: AstraZeneca Consulting or Advisory Role: Bristol-Myers Squibb Research Funding: Merck, Janssen, Becton Dickinson, United Health Group Patents, Royalties, Other Intellectual Property: Invention disclosure titled "Methods and Compositions for Treating Neuroblastoma", and invention disclosure titled "Methods and Compositions for Identifying, Diagnosing and Treating Neuroblastoma" Travel, Accommodations, Expenses: AstraZeneca, Foundation Medicine,Katie QuinnEmployment: Guardant Health Stock and Other Ownership Interests: Guardant HealthCarin R. EspenschiedEmployment: Guardant Health Stock and Other Ownership Interests: Guardant HealthAllysia MakEmployment: Guardant Health Stock and Other Ownership Interests: Guardant HealthLesli A. KiedrowskiEmployment: Guardant Health Stock and Other Ownership Interests: Guardant HealthMartina LefterovaEmployment: Guardant Health Stock and Other Ownership Interests: Guardant Health Consulting or Advisory Role: PersonalisRebecca J. NagyEmployment: Guardant Health Stock and Other Ownership Interests: Guardant HealthSharyn I. KatzConsulting or Advisory Role: Trizell Research Funding: NovartisChristine A. CiunciHonoraria: Imedex Research Funding: Celgene, Merck, Bristol-Myers Squibb, MacroGenicsJoshua M. BaumlConsulting or Advisory Role: Bristol-Myers Squibb, Merck, AstraZeneca, Genentech, Celgene, Boehringer Ingelheim, Guardant Health, Takeda, Novartis, Janssen, Ayala Pharmaceuticals, Regeneron, Inivata, Foundation Medicine Research Funding: Merck, Carevive Systems, Novartis, Incyte, Bayer, Janssen, AstraZeneca, Takeda, Amgen, Pfizer, Mirati TherapeuticsRoger B. CohenConsulting or Advisory Role: Heat Biologics, Innate Pharma, Cantargia AB, Genocea Biosciences, AstraZeneca Research Funding: Heat Biologics, Merck, Celldex, Innate Pharma, Kyn therapeutics, Xencor, Genocea Biosciences Travel, Accommodations, Expenses: Heat Biologics, Innate Pharma, Genocea BiosciencesCorey J. LangerHonoraria: Bristol-Myers Squibb, Genentech/Roche, Lilly/ImClone, AstraZeneca, Takeda Science Foundation, Merck Consulting or Advisory Role: Genentech/Roche, Lilly/ImClone, Merck, Abbott Biotherapeutics, Bayer/Onyx, Clarient, Clovis Oncology, Celgene, Cancer Support Community, Bristol-Myers Squibb, ARIAD, Takeda, AstraZeneca, Pfizer, Novocure, Gilead Sciences Research Funding: Merck, Advantagene, Clovis Oncology, Celgene, Inovio Pharmaceuticals, Ariad, GlaxoSmithKline, Genentech/Roche, Stem CentRx, Lilly, Trizell Other Relationship: Lilly, Amgen, Peregrine Pharmaceuticals, SyntaCharu AggarwalConsulting or Advisory Role: Genentech, Lilly, Celgene, Merck, AstraZeneca Speakers' Bureau: AstraZeneca, Roche/Genentech, An Immediate Family Member Research Funding: Genentech/Roche, Incyte, Macrogenics, Merck Sharp & Dohme, AstraZeneca/MedImmune No other potential conflicts of interest were reported.
Figures






References
-
- Mok TSK, Wu YL, Kudaba I, et al. : Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393:1819-18302019 - PubMed
-
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. : Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078-20922018 - PubMed
-
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-18332016 - PubMed
-
- Paz-Ares L, Luft A, Vicente D, et al. : Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040-20512018 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

