Aspirin at 120: Retiring, recombining, or repurposing?
- PMID: 34095732
- PMCID: PMC8162399
- DOI: 10.1002/rth2.12516
Aspirin at 120: Retiring, recombining, or repurposing?
Abstract
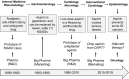
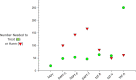
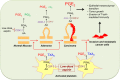
During the past 20 years, we have witnessed the following trends in aspirin usage: (i) a "dropping" trend, characterized by the early discontinuation of low-dose aspirin from dual antiplatelet therapy or triple antithrombotic therapy (oral anticoagulation plus dual antiplatelet therapy in patients with atrial fibrillation) following an acute coronary syndrome or after percutaneous coronary intervention; (ii) a "combinatorial" trend, featuring the addition of a lower dose of a P2Y12 inhibitor or direct oral anticoagulant drug to low-dose aspirin for the long-term treatment of stable patients with atherosclerotic cardiovascular disease; and (iii) a "repurposing" trend, characterized by growing interest in the oncologic community to assess the chemopreventive effect of aspirin against certain types of cancers (particularly of the gastrointestinal tract), both as primary prevention and adjuvant therapy. The aim of this review is to present the mechanistic rationale underlying these trends, discuss the design and findings of trials testing novel treatments or new therapeutic applications of aspirin, and report on the ISTH Congress results on this topic.
Keywords: P2Y12 inhibitors; aspirin; cardiovascular disease; colorectal cancer; nonsteroidal anti‐inflammatory drugs; oral anticoagulants.
© 2021 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures
Similar articles
-
The role of triple antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention.Prog Cardiovasc Dis. 2021 Nov-Dec;69:11-17. doi: 10.1016/j.pcad.2021.11.010. Epub 2021 Dec 6. Prog Cardiovasc Dis. 2021. PMID: 34883097 Review.
-
Managing antithrombotic therapy in patients with both atrial fibrillation and coronary heart disease.Clin Ther. 2014 Sep 1;36(9):1176-81. doi: 10.1016/j.clinthera.2014.08.010. Clin Ther. 2014. PMID: 25234549 Review.
-
Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials.Europace. 2020 Jan 1;22(1):33-46. doi: 10.1093/europace/euz259. Europace. 2020. PMID: 31603196
-
The Role of Double and Triple Therapy with Direct Oral Anticoagulants in Coronary Artery Disease, Peripheral Artery Disease, and Stroke.Clin Ther. 2018 Nov;40(11):1907-1917.e3. doi: 10.1016/j.clinthera.2018.09.014. Epub 2018 Oct 24. Clin Ther. 2018. PMID: 30458931
-
Risk of Stroke vs. Intracerebral Hemorrhage in Patients with Non-Valvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials Comparing Dual vs. Triple Antithrombotic Therapy.J Stroke Cerebrovasc Dis. 2021 Apr;30(4):105654. doi: 10.1016/j.jstrokecerebrovasdis.2021.105654. Epub 2021 Feb 10. J Stroke Cerebrovasc Dis. 2021. PMID: 33578352
Cited by
-
Drug repurposing: Clinical practices and regulatory pathways.Perspect Clin Res. 2025 Apr-Jun;16(2):61-68. doi: 10.4103/picr.picr_70_24. Epub 2024 Sep 10. Perspect Clin Res. 2025. PMID: 40322475 Free PMC article. Review.
-
Review of Under-Recognized Adjunctive Therapies for Cancer.Cancers (Basel). 2022 Sep 29;14(19):4780. doi: 10.3390/cancers14194780. Cancers (Basel). 2022. PMID: 36230703 Free PMC article. Review.
-
Low-dose aspirin for the prevention of atherosclerotic cardiovascular disease.Eur Heart J. 2024 Jul 12;45(27):2362-2376. doi: 10.1093/eurheartj/ehae324. Eur Heart J. 2024. PMID: 38839268 Free PMC article. Review.
-
Needs-based considerations for the role of low-dose aspirin along the CV risk continuum.Am J Prev Cardiol. 2024 Apr 15;18:100675. doi: 10.1016/j.ajpc.2024.100675. eCollection 2024 Jun. Am J Prev Cardiol. 2024. PMID: 38694728 Free PMC article.
-
Stability of the thromboxane B2 biomarker of low-dose aspirin pharmacodynamics in human whole blood and in long-term stored serum samples.Res Pract Thromb Haemost. 2024 Nov 12;8(8):102623. doi: 10.1016/j.rpth.2024.102623. eCollection 2024 Nov. Res Pract Thromb Haemost. 2024. PMID: 39698184 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

