Dravet syndrome-associated mutations in GABRA1, GABRB2 and GABRG2 define the genetic landscape of defects of GABAA receptors
- PMID: 34095830
- PMCID: PMC8176149
- DOI: 10.1093/braincomms/fcab033
Dravet syndrome-associated mutations in GABRA1, GABRB2 and GABRG2 define the genetic landscape of defects of GABAA receptors
Erratum in
-
Correction to: Dravet syndrome-associated mutations in GABRA1, GABRB2 and GABRG2 define the genetic landscape of defects of GABAA receptors.Brain Commun. 2022 Jun 24;4(3):fcac156. doi: 10.1093/braincomms/fcac156. eCollection 2022. Brain Commun. 2022. PMID: 35770130 Free PMC article.
Abstract
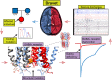
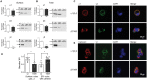
Dravet syndrome is a rare, catastrophic epileptic encephalopathy that begins in the first year of life, usually with febrile or afebrile hemiclonic or generalized tonic-clonic seizures followed by status epilepticus. De novo variants in genes that mediate synaptic transmission such as SCN1A and PCDH19 are often associated with Dravet syndrome. Recently, GABAA receptor subunit genes (GABRs) encoding α1 (GABRA1), β3 (GABRB3) and γ2 (GABRG2), but not β2 (GABRB2) or β1 (GABRB1), subunits are frequently associated with Dravet syndrome or Dravet syndrome-like phenotype. We performed next generation sequencing on 870 patients with Dravet syndrome and identified nine variants in three different GABRs. Interestingly, the variants were all in genes encoding the most common GABAA receptor, the α1β2γ2 receptor. Mutations in GABRA1 (c.644T>C, p. L215P; c.640C>T, p. R214C; c.859G>A; V287I; c.641G>A, p. R214H) and GABRG2 (c.269C>G, p. T90R; c.1025C>T, p. P342L) presented as de novo cases, while in GABRB2 two variants were de novo (c.992T>C, p. F331S; c.542A>T, p. Y181F) and one was autosomal dominant and inherited from the maternal side (c.990_992del, p.330_331del). We characterized the effects of these GABR variants on GABAA receptor biogenesis and channel function. We found that defects in receptor gating were the common deficiency of GABRA1 and GABRB2 Dravet syndrome variants, while mainly trafficking defects were found with the GABRG2 (c.269C>G, p. T90R) variant. It seems that variants in α1 and β2 subunits are less tolerated than in γ2 subunits, since variant α1 and β2 subunits express well but were functionally deficient. This suggests that all of these GABR variants are all targeting GABR genes that encode the assembled α1β2γ2 receptor, and regardless of which of the three subunits are mutated, variants in genes coding for α1, β2 and γ2 receptor subunits make them candidate causative genes in the pathogenesis of Dravet syndrome.
Keywords: Dravet syndrome-associated mutations; GABRA1; GABRB2; GABRG2; PIP2.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures






Similar articles
-
Association of nonsense mutation in GABRG2 with abnormal trafficking of GABAA receptors in severe epilepsy.Epilepsy Res. 2014 Mar;108(3):420-32. doi: 10.1016/j.eplepsyres.2013.12.005. Epub 2014 Jan 3. Epilepsy Res. 2014. PMID: 24480790
-
Mutant GABA(A) receptor subunits in genetic (idiopathic) epilepsy.Prog Brain Res. 2014;213:55-85. doi: 10.1016/B978-0-444-63326-2.00003-X. Prog Brain Res. 2014. PMID: 25194483 Review.
-
Overexpressing wild-type γ2 subunits rescued the seizure phenotype in Gabrg2+/Q390X Dravet syndrome mice.Epilepsia. 2017 Aug;58(8):1451-1461. doi: 10.1111/epi.13810. Epub 2017 Jun 6. Epilepsia. 2017. PMID: 28586508 Free PMC article.
-
Three epilepsy-associated GABRG2 missense mutations at the γ+/β- interface disrupt GABAA receptor assembly and trafficking by similar mechanisms but to different extents.Neurobiol Dis. 2014 Aug;68:167-79. doi: 10.1016/j.nbd.2014.04.015. Epub 2014 May 4. Neurobiol Dis. 2014. PMID: 24798517 Free PMC article.
-
Molecular Pathogenic Basis for GABRG2 Mutations Associated With a Spectrum of Epilepsy Syndromes, From Generalized Absence Epilepsy to Dravet Syndrome.JAMA Neurol. 2016 Aug 1;73(8):1009-16. doi: 10.1001/jamaneurol.2016.0449. JAMA Neurol. 2016. PMID: 27367160 Free PMC article. Review.
Cited by
-
Pharmacological chaperones restore proteostasis of epilepsy-associated GABAA receptor variants.Pharmacol Res. 2024 Oct;208:107356. doi: 10.1016/j.phrs.2024.107356. Epub 2024 Aug 30. Pharmacol Res. 2024. PMID: 39216838 Free PMC article.
-
Epileptic Encephalopathy GABRB Structural Variants Share Common Gating and Trafficking Defects.Biomolecules. 2023 Dec 14;13(12):1790. doi: 10.3390/biom13121790. Biomolecules. 2023. PMID: 38136660 Free PMC article.
-
Pathogenic variants of human GABRA1 gene associated with epilepsy: A computational approach.Heliyon. 2023 Sep 15;9(9):e20218. doi: 10.1016/j.heliyon.2023.e20218. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809401 Free PMC article.
-
Integrated Proteotranscriptomics of the Hypothalamus Reveals Altered Regulation Associated with the FecB Mutation in the BMPR1B Gene That Affects Prolificacy in Small Tail Han Sheep.Biology (Basel). 2022 Dec 30;12(1):72. doi: 10.3390/biology12010072. Biology (Basel). 2022. PMID: 36671764 Free PMC article.
-
GABRG2 Variants Associated with Febrile Seizures.Biomolecules. 2023 Feb 22;13(3):414. doi: 10.3390/biom13030414. Biomolecules. 2023. PMID: 36979350 Free PMC article.
References
-
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676–685. - PubMed
-
- Katsnelson A, Buzsaki G, Swann JW.. Catastrophic childhood epilepsy: A recent convergence of basic and clinical neuroscience. Sci Transl Med. 2014;6(262):262ps13. - PubMed
-
- Marini C, Scheffer IE, Nabbout R, et al. The genetics of Dravet syndrome. Epilepsia. 2011;52 (Suppl 2):24–29. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
